Montag, 25. April 2016
Plant Extracts 101(Part IV): Phytochemicals in cosmetics: Terpenoids
If you have already read part I, Part II and part III of our "plant extract 101", you already know that the selection of the carrier or the extract type mainly depends on your desired active ingredients.
Whatever your aim of extraction might be:
- Do you want to have the colour of a certain plant (beetroot or turmeric for instance)?
- Do you want to incorporate the scent of a certain plant part in your product (vanilla or coffee for example)?
- Are you looking for certain active/therapeutic ingredients in a given plant (Sterols, vitamins, flavonoids, anti-oxidants etc.)?
You're going to extract different phytochemicals and knowing in advance about the phytochemicals you shall extract helps you find a soluble carrier and probably the best (most efficient) way of extraction.
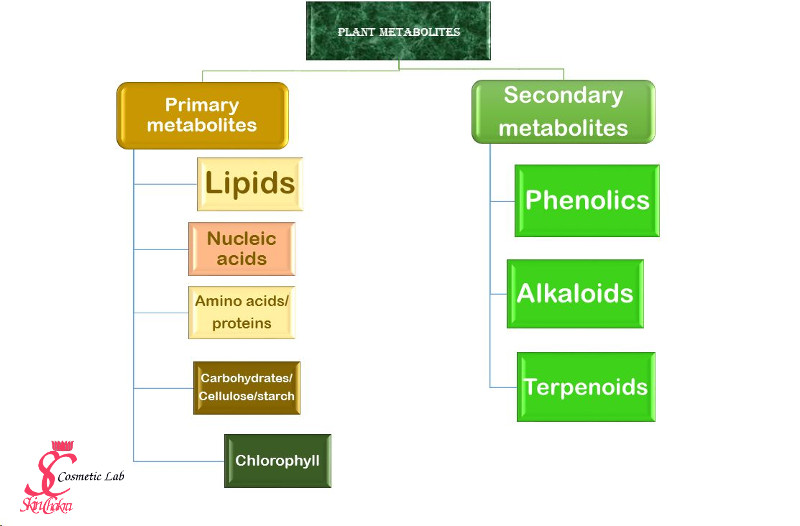
If you can remember from the last part, secondary metabolites (chemicals that are not vital for plant nutrition and growth) are classified into three main categories:
We've briefly mentioned alkaloids in the last part and would not dedicate more time to them. These are basically poisonous and stimulant material most of them being applied in pharmacy and in insecticide, pesticide industry.
In this part, we're going to discuss one of the most interesting group of secondary metabolites, the terpenoids.
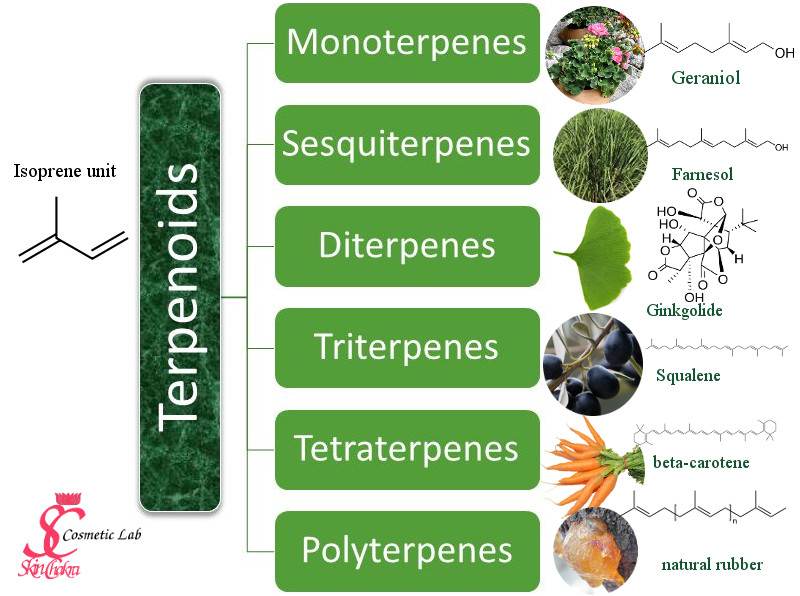
Terpenoids have the backbone of isoprene, a hydrocarbon with 5 carbon atoms (C5). This is the starting material for thousands of amazing ingredients with a very broad spectrum of properties and applications.
Monoterpenes
You are probably familiar with monoterpenes from essential oils. These are light hydrocarbons, consisting of two isoprene units (C10H16) which are soluble in alcohol or an oil. Monoterpenes are present in many distilled or pressed essential oils.
The most prominent examples are: Geraniol (monoterpenol) from rose or geranium essential oil, Limonene from citrus essential oils, linalool (monoterpenol) from lavendder essential oil.
Monoterpenes have different properties ranging from anti-bacterial1 to cancer prevention2. Other properties include antinociceptive3 (reducing sensitivity and pain) and antifungal4 properties.
Sesquiterpenes
Sesquiterpenes consist of three isoprene units (C15H24). The molecules are still light enough to be distilled in the essential oils. The most prominent members include farnesol (jasmine, rose, ylang-ylang and beta-bisabolene (basil and oregano).
Sesquiterpenes have antifungal, antibacterial and anaesthetic5 as well as antioxidant, anti-inflammatory6 and deodorant properties.
Diterpenes
Diterpenes consist of 4 isoprene units (C20H32). The molecule is too heavy to be distilled and diterpenes are usually found in resinoid essential oils (if at all). Ginkgolides are diterpenes extracted Ginkgo biloba7 leaves. They are used in pharmacy in cardiovascular treatments8 and in migraine9 treatment drugs as well as in drugs against dementia and Alzheimer's10. Cafestol and kahweol are diterpenes from coffee with anticancer properties11.
Triterpenes
They consist of 6 isoprene units (C30H48). Steroids are derivatives of triterpenes and Saponis (plant based surfactants) belong either to triterpene or steroid sub-categories.
Squalene is the most prominent member of this group. Since squalene has a high Iodine value (and a low shelf-life) it is hydrogenated to squalane, the very popular emollient which most of you apply in your cosmetics (it could be either derived from olive, amaranth, wheat germ oil or from whale liver oil).
All those amazing phytosterols from plant oils and extracts belong to this category. They perform anti-oxidant, anti-ageing and anti-inflammatory properties. Sitosterol, campesterol and stigmasterol are the most prominent members of this group. They are found in unrefined plant oils such as wheat germ oil, rice bran oil, soybean oil, macadamia oil and olive oil.
Tetraterpenes
These are represented by carotenes. Tetraterpenes consist of 8 isoprene units (C40H64). These molecules are big and have a significant colour. Lycopene (from tomato) and crocin (from saffron) belong to tetraterpenes.
In skin care they perform photoprotective12, antioxidant13 and anti-inflammatory properties.
Polyterpenes
Polyterpenes consist of hundreds of isoprene units. Natural rubber is the most prominent member of this group. Latex which is synthesized in vesicles of laticifers (latex bearing plants) is another member of polyterpenes. These are insignificant for our purposes. You shall only take care of possible latex allergy when applying certain plant oils (such as avocado oil) in your formulations.
Well, you may now ask: so what?
Knowing the lipophilic characteristics of terpenoids (and alcohol soluble for the smaller mono- and sesqui-terpenoids), you've realized that when you want any of these ingredients in your skin care products, you'll need a lipophilic extract or a tincture (hydro-alcoholic) extract. There is no way of having farnesol, lycopene or beta-carotene in a hydrophilic extract.
I hope you'll find this post helful and useful for application of extracts in your cosmetic formulations. Feel free to share this post with your friends and colleagues and feel free to send me your questions and comments. I promise to answer them in a couple of days.I really enjoy your participation in our Facebookdiscussions.
Proceed to the next part (polyphenols).
Be Happy and have fun
References:
1- Kotan, Recep, Saban Kordali, and Ahmet Cakir. "Screening of antibacterial activities of twenty-one oxygenated monoterpenes." Zeitschrift für Naturforschung C 62.7-8 (2007): 507-513.
2- Gould, Michael N. "Cancer chemoprevention and therapy by monoterpenes."Environmental Health Perspectives 105.Suppl 4 (1997): 977.
3- Liapi, Charis, et al. "Antinociceptive properties of 1, 8-Cineole and beta-pinene, from the essential oil of Eucalyptus camaldulensis leaves, in rodents." Planta medica 73.12 (2007): 1247-1254.
4- Hammer, KA 1., C. F. Carson, and T. V. Riley. "Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil." Journal of Applied Microbiology 95.4 (2003): 853-860.
5- Dolara, Piero, et al. "Local anaesthetic, antibacterial and antifungal properties of sesquiterpenes from myrrh." Planta medica 66.4 (2000): 356-358.
6- Tang, Wen-Zhao, et al. "New sesquiterpene lactone and neolignan glycosides with antioxidant and anti-inflammatory activities from the fruits of Illicium oligandrum." Planta medica 73.5 (2007): 484-490.
7- Van Beek, T. A., et al. "Determination of ginkgolides and bilobalide in Ginkgo biloba leaves and phytopharmaceuticals." Journal of Chromatography A 543 (1991): 375-387.
8- Koch, E. "Inhibition of platelet activating factor (PAF)-induced aggregation of human thrombocytes by ginkgolides: considerations on possible bleeding complications after oral intake of Ginkgo biloba extracts." Phytomedicine 12.1 (2005): 10-16.
9- Esposito, Maria, and Marco Carotenuto. "Ginkgolide B complex efficacy for brief prophylaxis of migraine in school-aged children: an open-label study."Neurological Sciences 32.1 (2011): 79-81.
10- Ahlemeyer, B., and J. Krieglstein. "Pharmacological studies supporting the therapeutic use of Ginkgo biloba extract for Alzheimer's disease."Pharmacopsychiatry 36 (2003): S8-14.
11- Cavin, C., et al. "Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity." Food and Chemical Toxicology 40.8 (2002): 1155-1163.
12- Gollnick, H. P. M., et al. "Systemic beta carotene plus topical UV-sunscreen are an optimal protection against harmful effects of natural UV-sunlight: results of the Berlin-Eilath study." EJD. European journal of dermatology 6.3 (1996): 200-205.
13-Greenberg, E. Robert, et al. "A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin." New England Journal of Medicine323.12 (1990): 789-795.