
For Part V click here please.
No matter whether you're interested in natural fats and oils as a soap maker, as a lotion maker, as an aromatherapist or simply from a nutritional point of view, there are certain characteristics which define the nature and the quality of the oil and which simply reveal if the oil is old, adulterated or has lost its quality during processing (in case of refined oils).
You may have often heard of: Acid value, saponification value, iodine value and peroxide value. The first three terms are probably more common than the last one, peroxide value. Let's have a short review on each of them and on their importance for you as a consumer.
Saponification value is probably the most familiar of all other terms, specially if you're a soap maker. Not only soap makers are interested and involved in saponification value however. This is one of several specification criteria for each fat (animal or plant based), oil, butter, wax and fatty acid and should be mentioned in each TDS.
Saponification value is the mg of KOH (Potassium hydroxide) required to saponify 1g of sample (fat, oil, butter or fatty acid). As soap makers we work with this term day in and day out. Saponification value of a fat, oil or butter is the stoichiometric sum of it's fatty acid spectrum. In simple words, if an oil is composed of 20% Lauric acid (C12:00), 50% myristic acid (C14:0), 10% Stearic acid (C18:0), 20% Oleic acid (C18:1), the sap. number would be the geometric sum of individual sap. numbers.
When making soap, we combine different oils and fats with different sap. numbers. The required amount of lye (NaOH or KOH) is then calculated as a geometric sum of the individual oils. Earlier we had to calculate the lye content manual and as a geometric sum. Today, there are wonderful programs, most of them available for free which do the same thing without you knowing the background of the calculation. Soapcalc for example is one of the most reliable programs available for free.
Sap. number is generally expressed as mg KOH. This is internationally accepted since the measurement is often run by a KOH solution in alcohol. Soapmakers however, require the amount of NaOH for lye-preparation and making of bar soaps. We have as well converted the lye amount from KOH to NaOH ourselves. The lye-calculation programs such as Soapcalc calculate the value both for NaOH and for KOH.
Sap. number or sap. value are characteristics of any specific oil, fat, butter or wax.
Generally, saponification value is determined by:
Boiling the sample with an alcoholic solution of KOH (in surplus). After the saponification is completed (ca. 1 hr) the surplus KOH would be titrated (measured) with HCl (hydrochloric acid). The end point could be determined by a chemical indicator (such as Phenolphthalein) or by colorimetry or potentiometry. There are other instrumental methods as well for determination of sap. value which are applied in oil plants. One of the most widely applied standard methods for determination of sap. value is the ASTM D5558 - 95(2011) Standard Test Method for Determination of the Saponification Value of Fats and Oils .
Saponification value is characteristic of the fatty acid spectrum of any fat, oil, butter or wax. Fatty acid spectrum could be slightly deviating regarding the harvest and the geographical origin of the oil. However it wouldn't change during storage, refining or processing.
Iodine value or iodine number is another characteristic of fats, oils, butters, fatty acids and waxes. As by sap. value, the iodine value is an stoichiometric or geometric sum of the constituent fatty acids. Iodine value is a characteristic of the unsaturated bonds in a fatty acid chain. The higher the concentration of unsaturated fatty acids in a given oil, the higher the iodine value and as a result the more susceptible that fat or oil to oxidation.
Iodine value is expressed as g I 2/100g of substance and is the amount of iodine required to oxidize double bonds in a given substance. Let's compare for example the iodine value of virgin coconut oil (7-11) with pomegranate seed oil (230-240). You realize the difference? Or the iodine value of virgin Soybean oil (125-140) and partially hydrated Soybean (78-90), in the latter part of the unsaturated bonds are hydrated. As a consequence, partially hydrated soybean oil has a pasty consistence @ room temperature, a lower iodine value and a longer shelf life.
Soapcalc is a wonderful source for iodine values of fats and oils applied in soap making. You may as well download our table showing the saponification value and the iodine number of the most common cosmetic- and soap making oils.
Iodine value is measured by Wijs method. The fat/oil/wax sample is reacted with a surplus of ICl (instead of IBr in the above diagram). The excess ICl is then back titrated and the amount of ICl required for oxidation of the double bonds is calculated.
ASTM D5554 - 95(2011) Standard Test Method for Determination of the Iodine Value of Fats and Oils is one of the most widely acknowledged standard methods for determination of iodine value in fats and oils.
Iodine value is expressed as a range because the fatty acid spectrum of fats and oils are highly dependent on harvest and geographical origin.
As like saponification value, iodine number is characteristic of the fatty acid spectrum of any fat, oil, butter or wax. Fatty acid spectrum could be deviating regarding the harvest and geographical origin of the oil having an impact on the iodine value. This is why iodine value is expressed as a range. Iodine value however, wouldn't change during storage or refining.
Acid value or acid number is a characteristic of fats, oils, butters and waxes which has a direct relation to a quality of the product. Acid value is the amount of free fatty acid present in a given sample. A high quality fat, oil butter or wax should contain as little fatty acid as possible (max. of 5%). Acid value is expressed as % or as the mg of KOH required to neutralize free fatty acid in 1 gr of a given sample. The lower the acid value the better or the more intact is the lipid or wax. With aging or because of poor quality of the processing (high temperature during extraction or refining) a part of the triglycerides may undergo lipolysis (breaking lipids) and fatty acids are set free, this is what we call rancidity.
Measurement of acid value is one of the simplest test to determine the quality of a given oil/wax. Free fatty acids are measured by a simple acid-base neutralisation. The end point being detected by a chemical indicator or by pH-metry/potentiometry. There are of course fully automated measurement systems applied in industrial plants.
ASTM D5555 - 95(2011) Standard Test Method for Determination of Free Fatty Acids Contained in Animal, Marine, and Vegetable Fats and Oils Used in Fat Liquors and Stuffing Compounds is an internationally acknowledged method for determination of free fatty acids.
Peroxide value is another characteristic directly related to the quality of the oil.
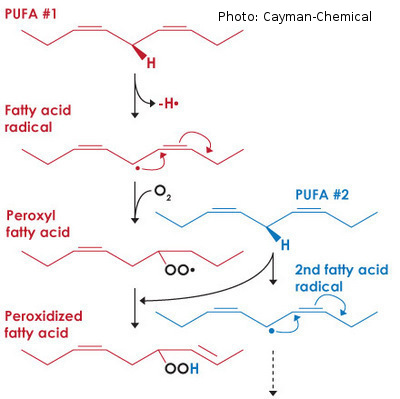
Generally, the higher the number of unsaturated C-C bonds (the higher the iodine value), the more susceptible the fatty acid against oxidation. Unsaturated fatty acids react with oxygen forming peroxides as a primary product. Peroxides accelerate other reactions causing ketones and fatty acids as end products (rancidity). Oxidation is accelerated by temperatures and by exposure to light and oxygen. The lower the peroxide and acid values, the better the quality of the specific sample.
Measurement of peroxide value:
The sample is treated in with a mixture of acetic acid and a suitable organic solvent and then with a solution
of potassium iodide. The liberated iodine is titrated with a standard solution of sodium thiosulfate
ISO 3960:2007 is an acknowledged method for determination of peroxide value.
Bottom line: Acid value and peroxide value reflect the quality of a given sample of oil/fat/wax. The higher the acid and the peroxide value the lower the quality of the given sample. The sample is either old, was subjected to oxygen and heat during storage or was poorly processed.
Iodine value and saponification value are related to the chemical composition of the oil/fat/wax. They do not change during storage or processing (except for hydrogenation).
Addition of antioxidants such as tocopherol to a given oil could prolong the shelf life. Antioxidants are radical scavengers and prevent oxidation (to a limited degree).
Swettis Beauty Blog am : Butter, fats, oils part V