Mittwoch, 27. April 2016
The confusing world of "natural waxes" in cosmetics (Part I)
Waxes (natural, synthetic or semi-synthetic) are applied in cosmetics fro many reasons:
- Improve texture
- Add shine
- Increase the viscosity
- Improve hardness
- Improve the stability of a product
- Increase/adjust the melting point
- Add hold
Are just a few applications. In addition to that, waxes are applied in huge volumes in depilation both in home applications as well as in professional salons. Depilatory waxes are however, basically based on paraffin which is cheap and stable but a no-go in "natural" cosmetics.
Bees wax is certainly the worlds most ancient wax being applied for thousands of years in candles, sacred incense and in cosmetics. It is still pretty much popular among traditional herbalists and DIY practitioners because of its availability and ease of working with.
There are however, many formulators (me included) who don't like the skin feel of the bees wax. In addition to that with the increasing demand for vegan cosmetics, the search for bees wax alternatives has been increased as well. There are tens of reasons why a modern organic formulator should be (at least on the theory) familiar with bees wax alternatives and plant based waxes for cosmetics application.
This is however easier to say than to achieve.
During the last couple of years I have been confronted with confused formulators asking about advantages and disadvantages of a certain wax for cosmetics application. These waxes have tempting and fanciful names but by looking into their INCI names one realizes that they are nothing more than a hydrogenated oil. There is nothing criminal about hydrogenating an oil, and some procedures (and subsequently the products) are even certified by certain organizations and associations such as NaTrue or Ecocert but a hydrogenated oil, is not (at least from a chemical point of view) a wax. It is, let's say comparable to vegetable shortenings or margarine in the kitchen. If you apply any of them in your kitchen and your nutrition, then probably you would have no objection to applying hydrogenated oils in your products. (And we all know that olive squalne is a hydrogenated form of squalene which is the natural ingredient derived from the olive oil).
Anyway, it is your legitimate preference to chose a real chemical wax or a hydrogenated oil in your products but to help hundreds of my confused readers know the difference, I'm dedicating a short post to the chemistry of waxes before introducing available plant based waxes for modern, sustainable cosmetic application in the coming post.
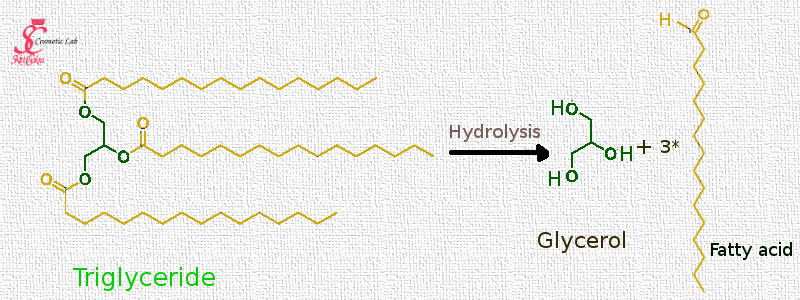
Let's start with triglycerides, or oils, fats and butters.
Oils, fats and butters are all considered triglycerides, or triacylglycerides (the left side structure). This is the same for plant based liquid oils such as avocado oil, plant based solid or semi solid oils such as coconut or babassu oil and butters such as cocoa or shea butter. Even animal based fats such as lard or duck fat have the same basic chemical structure: Triacylglyceride
These triglycerides consist of one molecule of glycerol (the green unit) attached to 3 unit of fatty acids (the yellow units) and these are the building blocks of fats and oils. Each fat, oil or butter consists of a variety of these triglycerides with the basic difference in the fatty acid chain.
As the triglyceride undergoes hydrolysis, the triglyceride breaks into a glycerine and 3 fatty acid molecules. This is very similar with the saponification reaction. In soap making, we break the triglyceride (fat, oil or butter) into a glycerine and 3 fatty acid salts (usually sodium salt because we use NaOH for saponification).
The fatty acid chains in a triglyceride could be all the same or different. In the above example, the triglyceride consists of 3- palmitin (C16:0). After hydrolysis, we'll have one glycerol and 3 palmitic acid units.
A fat, oil or butter consists of a range of different triglycerides with different fatty acid chains.
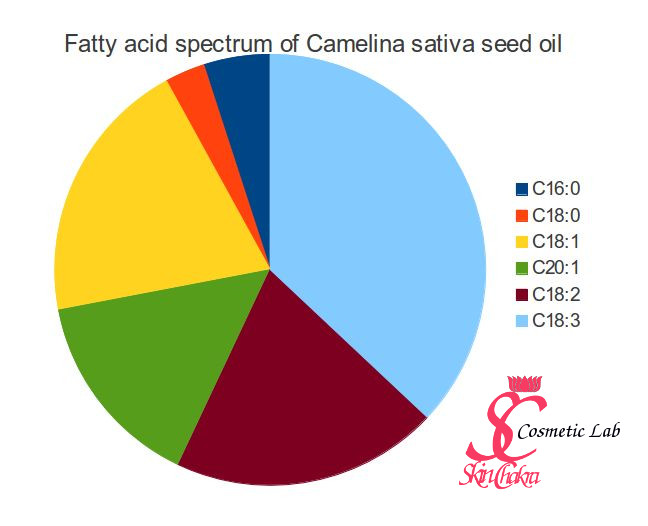
Look at the fatty acids in camelina sativa seed oil for example. The main constituent is C18:3, a polyunsaturated fatty acid, alpha-linolenic acid (which is a omega-3 fatty acid).
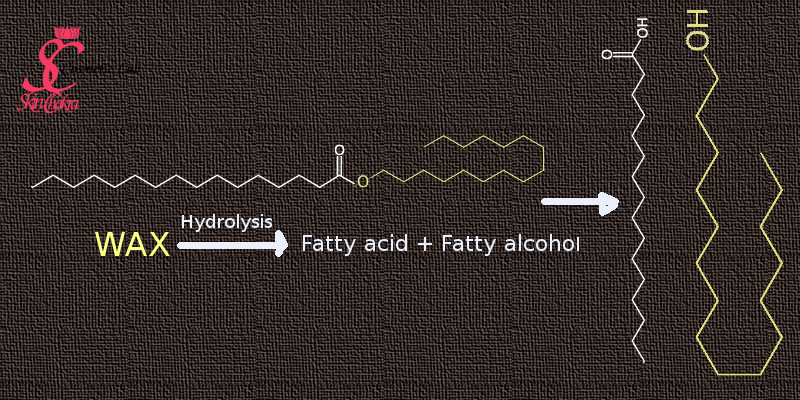
A wax, on the other hand, is basically consisting of fatty acid esters: a marriage between a fatty acid and a fatty alcohol.
In the above example, our wax consists of palmitic acid and cetyl alcohol, both consisting of a C16 saturated chain. Upon hydrolysis, this wax (cetyl palmitate) breaks into its building blocks, palmitic acid and cetyl alcohol. Just like plant oils, waxes (plant based, animal based or synthetic) are a combination of many ingredients but the main cosntituents are fatty esters (mono-, di- and tri-esters), long chain fatty alcohols, fatty acids and long chain hydrocarbons (named as well paraffinic hydrocarbons). The wax is separated/extracted as it is an undergoes some physical filtration, deodorization (and in some cases bleaching to achieve a wax with a decent color). There are no hydrogenation reactions involved.
Bees wax for example, consists basically of esters of long chain fatty alcohols (C30-C32) with palmitic acid (C16:0), palmitoleic acid (C16:1) and oleic acid (C18:1). Cerotic acid is another main constituent. It is, from a chemical point of view a real wax with a fatty ester structure.
The non-real wax, or hydrogenated (hardened) oil on the other hand, has a triglyceride structure. There are no esters in the hardened oils.
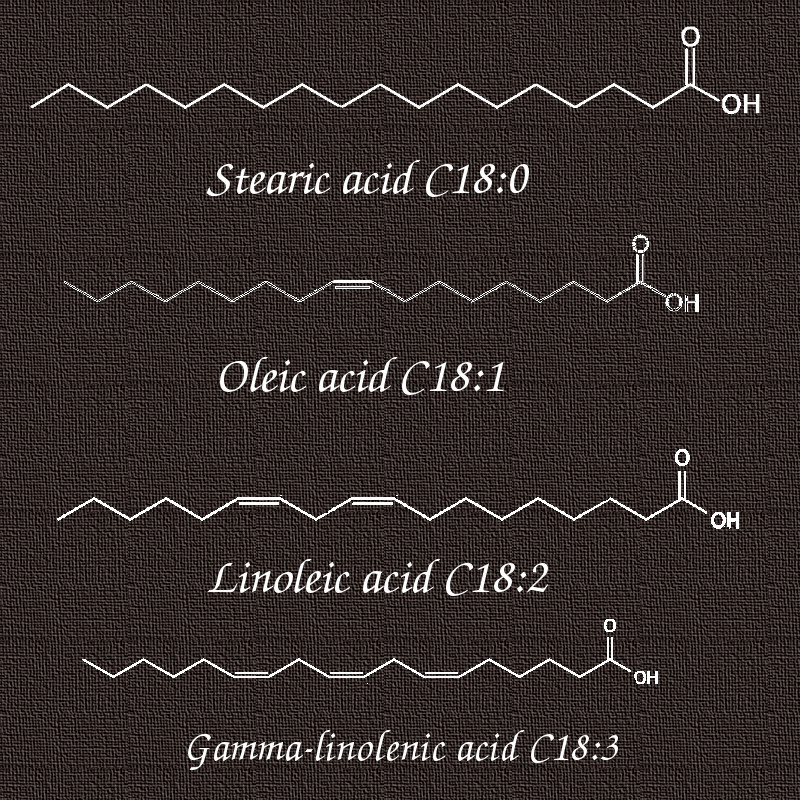
Have a look at the structure of stearic acid, oleic acid, linoleic and gamma-linolenic acid in the following picture.
They all consist of a C18 chain with the difference in hydrocarbon chain saturation. In stearic acid, the hydrocarbon chain is fully saturated (C18:0), oleic acid is a mono-unsaturated fatty acid (C18:1), linoleic acid is a double unsaturated fatty acid (C18:2) and gamma linolenic acid is a triple unsaturated fatty acid (C18:3).
As you certainly know, stearic acid is solid at room temperature whereas the other 3 fatty acids are liquid at room temperature.
Now, do you know what happens when we saturate these double bonds in linolenic, linoleic and oleic acid?
Bravo, these acids turn to stearic acid which is solid at room temperature. This is exactly what happens to hydrogenated (hardened) oils. Only in that case, we're not working with a single fatty acid but with a range of fatty acids with different chain lengths and different unsaturation grades.
The non-real plant waxes
Theoretically, all oils could be partially or completely hydrogenated to create a soft or hard solid product.
These are the ones commercially available that you've probably observed (or purchased) as plant based waxes:
| Soybean wax | HYDROGENATED SOYBEAN OIL |
| Soybean wax | HYDROGENATED SOY GLYCERIDES |
| Rapeseed wax | HYDROGENATED RAPESEED OIL |
| Rapeseed wax | HYDROGENATED RAPESEED GLYCERIDES |
| Castor wax | HYDROGENATED CASTOR OIL |
| Vegetable wax | HYDROGENATED VEGETABLE OIL |
| Olive wax | HYDROGENATED OLIVE OIL |
| Jojoba wax* | HYDROGENATED JOJOBA OIL |
| Hemp wax | HYDROGENATED HEMP SEED OIL |
* Jojoba oil is, from a chemical point of view, a natural and liquid wax (esters instead of triglycerides). The liquid is then hydrogenated to form the solid which is often supplied as jojoba beads (usually as a natural exfoliant).
These are some of the most common, but certainly not all of the hydrogenated oils being offered as plant based waxes. In case of any doubt, look at the INCI name of the product which should clearly indicate whether it is a real wax or a hydrogenated oil.
In part II of this post, I'll introduce some of real natural waxes I've worked with.
Thank you for your visit. Feel free to send me your questions, comments and suggestions. I really enjoy your participation in our Facebook discussions.