Montag, 27. Juni 2016
How to protect your "natural" preservatives from deactivation
"Natural" preserving of cosmetics is a hot and basic topic in the cosmetic industry. Not only the "organic" cosmetic industry and the "organic" consumer is constantly in search of "milder" and "more natural" preservatives, even the mainstream industry ins trying to appear just a little bit more "natural" and "consumer friendly" by making "paraben-free", "preservative-free" or "natural preservative" claims.
If you're not quite familiar with the application of "natural" preservatives, the global legislative issues and the GMP, please have a look at one of my best sold courses "Certificate in natural cosmetic preservation". We've originally offered the course only twice a year but now and because of the increasing demand, it is offered as an 2on demand" course.
If you are familiar with some "natural" preservatives you know that most of these preservatives (both those who are officially listed in the EU list of approved preservatives and those who are not listed as preservatives) belong to the "weak organic acid" group.
To this group belong:
- Benzoic acid/ sodium benzoate
- Sorbic acid/ potassium sorbate
- Salicylic acid
- DHA (dehydroacetic acid) and its sodium salt
- Levulinic acid/ sodium levulinate
- p-anisic acid and its sodium salt
- Glucuronic acid
These acids are quite challenging to the formulator. Generally a "natural" product containing plant oils, extracts, honey, natural gums and other challenging and "difficult to preserve" ingredients is much more difficult to preserve compared to a mainstream and conventional product. By applying these "natural & mild" preservatives, the task of preservation and the requirements for penible hygienic procedure and meticulous GMP is even more important than when applying broad spectrum and "conventional" preservatives blends.
Knowing that most of these preservatives are more expensive and their application dosage is higher compared to the "conventional" preservatives, any "natural" formulator is interested to "get the best of" her preservatives, both for the sake of the budget and for protecting the product and the customer.
One of the main technical challenges when working with these preservatives is the pH adjustment. This is why most reliable suppliers of "natural preservatives" mention an exact range for the efficacy of any given preservative. There are however some fanciful retailers who boldly mention a broad pH range of 3-8 for a preservative blend containing at least one of these weak acids. This is not only misleading, it could even be dangerous to the consumer.
- Suppose you apply a blend of preservatives and do not exactly adjust the pH in the required range where your preservative has the max. efficacy. In this case, you're both wasting a part of your preservative and endangering the integrity of the product and safety of the consumer.
- It could be even worse. Suppose you adjust the pH of the product exactly in the max. efficacy range but the pH changes during the storage and application (meaning the product is unstable). With the pH being changed the efficacy of the preservative is reduced without you ever noticing it or having any control over it because the product has left your premises. You can read our earlier post about the importance of stability testing of your cosmetic products here.
Why is the pH so important when working with "natural" preservatives and "weak acids"?
These weak acids are only active and effective in their acidic form and not in their salt form. It means when you apply sodium benzoate or potassium sorbate or any of the preservatives in the above list, they should be in their acidic form otherwise they are less effective or absolutely non-effective.
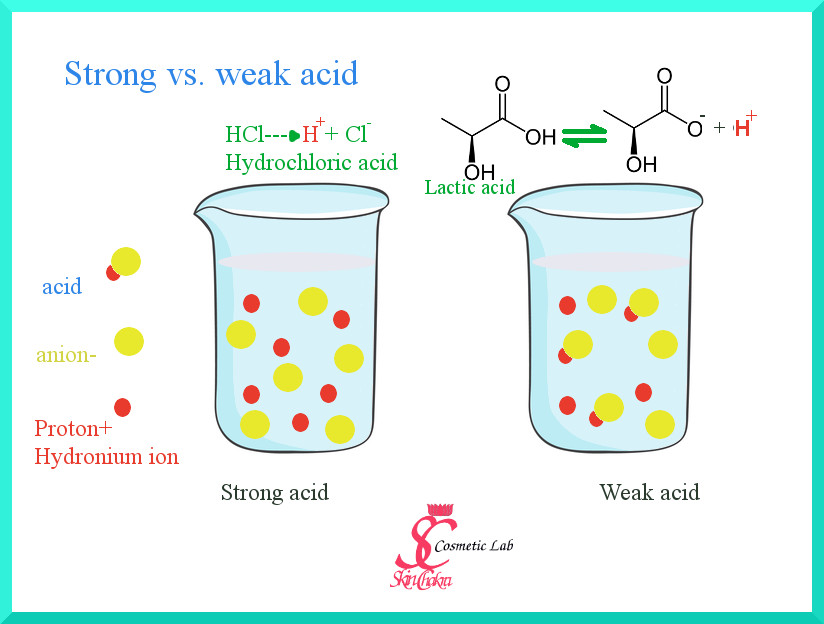
Let's first explain a little bit about the weak acids for those of you who have day dreamed during chemistry course at the high school. 
A strong acid such as hydrochloric acid, is fully dissociated in water. It means it is completely formed into hydronium cations (H+ or H3O+) and chloride anions (Cl-).
A weak acid such as lactic acid however, does not completely dissociate in water. It is only partially dissociated to the hydronium cation and lactate anion which coexist together with some undissociated lactic acid.
Each weak acid has a dissociation constant (Ka). How much of the acid is dissociated and how much is in the undissociated form is determined by the acidity (or dissociation constant) and by pH. It means, that for each weak acid the degree of dissociation changes according to pH.
As a very general rule, at a pH=pKa exactly 50% of the acid is in dissociated form and 50% is in undissociated form. At a pH lower than the pKa, more acid is in undissociated form and vice versa at a pH higher than pKa, more acid is in dissociated form.
Let's make an example for a better understanding:
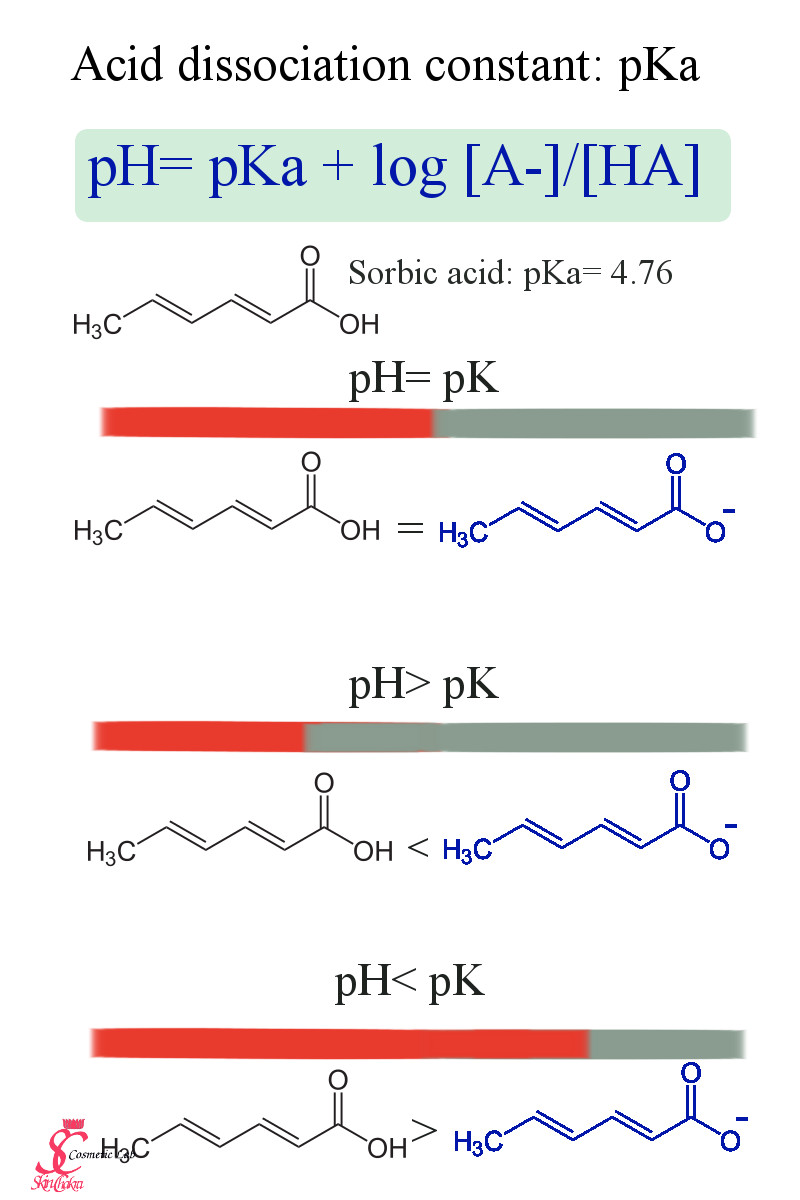
The above diagram depicts the acid dissociation constant for sorbic acid (which is one of the common natural preservatives).
Sorbic acid has a dissociation constant of 4.76. It means that at a pH= 4.76 exactly 50% of acid is is in acid form, at a pH higher than 4.76, less than 50% is in acid form and at a pH lower than 4.76 more than 50% is in acid form. (Hopefully this makes sense and doesn't confuse you too much).
This means, the efficacy of a preservative based on sorbic acid would vary according to pH. At a pH of 4.76 sorbic acid os almost 100% efficient. By increasing pH, the efficacy would reduce drastically. This is the reason why all of preservatives based on weak organic acids (almost all natural preservatives) have an optimum pH range. In order to keep the preservative efficient, it is extremely important to adjust the pH of the product and to make sure the pH doesn't fall out of the efficacy range during the whole shelf-life with suitable stability tests.
Let's make some examples so that this dry-chemistry stuff becomes more comprehensible.
The following table depicts the efficacy of the 7 weak acids mentioned earlier in this post. At least one of these acids (or its salt) are found in almost all modern "natural" preservatives.
| pKa | pH=3 | pH=4 | pH=5 | pH=6 | pH=7 | |
| Benzoic acid | 4.18 | 94% | 61% | 13% | 1.5% | 0% |
| Sorbic acid | 4.76 | 98% | 85% | 37% | 5.5% | 0% |
| Salicylic acid | 2.97 | 48% | 9% | 1% | 0% | 0% |
| DHA | 5.27 | 100% | 95% | 65% | 16% | 2% |
| Levulinic acid | 4.5 | 97% | 76% | 24% | 3% | 0% |
| p-anisic acid | 4.47 | 97% | 75% | 23% | 3% | 0% |
| Glucuronic acid | 3.7 | 83% | 33% | 5% | 0.5% | 0% |
Studying the above table could be quite intimidating. Look how each of these preservatives loose their efficacy as the pH increases. One simple response could be: reducing the pH to as low as possible (which is, for most skincare products about 4.5).
Well, the simplest solution is not always the best solution.
For almost all of the above acids, the solubility of the acid form is very limited in water. Have you ever tries to dissolve salicylic acid powder in water? It doesn't dissolve until you increase the pH of the water. By increasing the pH, you transform the acid to its sodium salt which has a superior solubility compared to teh acid.
This is why most of these acids are offered as the sodium or potassium salt in preservative blends: sodium benzoate, potassium sorbate, sodium levulinate etc.
Only the acid form is effective but the acid form has a very low solubility in water
It sounds quite trifling doesn't it?
You can observe this problem in the above picture. The samples are micellar water with a pH around 4.8-5.2 which is quite a reasonable pH range for micellar waters but not for many of these "natural" preservative acids. By these samples, the micellar water was quite clear at room temperature and thise turbidity/crystalization occured in the fridge after a few hours.
This is another important reason to stability test your cosmetic products.
The sedimentation/crystalization of the preservative is not only damaging the appearance of the product, it is dangerous because your preservative is kicked out of the solution. It means your product would be left unguarded to microorganisms to have a jolly party.
In the above example, you can easily observe that "something" is not all right but suppose you're working with an emulsion of a high viscosity cream or any other opaque/turbid system where you can not even observe the sedimentation of the preservative. Unless you run challenge tests during the stability testing and storage of the product, you wouldn't even realize that your product is becoming "unpreserved" and is inviting micro-organisms.
Isn't that quite scary?
Well to avoid such unwanted changes in your product you have to (at least):
- run meticulous stability tests including challenge tests during your product development phase
- run stability tests for each production batch, tracking any pH changes and changes in viscosity, density and appearance of samples kept at different temperatures (at least reduced temperature, ambient temperature and elevated temperature)
- determine the solubility range of your preservative system at different temperatures over the efficacy pH range
By optimizing the pH range considering the solubility and the efficacy of the preservative you can get the best of any given preservative without compromising stability, appearance or microbiological stability of the product and without wasting or overdosing your preservative.
Now that the importance of pH adjustment is clear to you, let us have a very simple look at the mechanism of action of these preservatives to know why they are effective as the acid form and why you shall adjust the pH so that the salt form is converted to the acid form.
Disclaimer: Excuse my poor design. I hope you can understand the principle anyway.
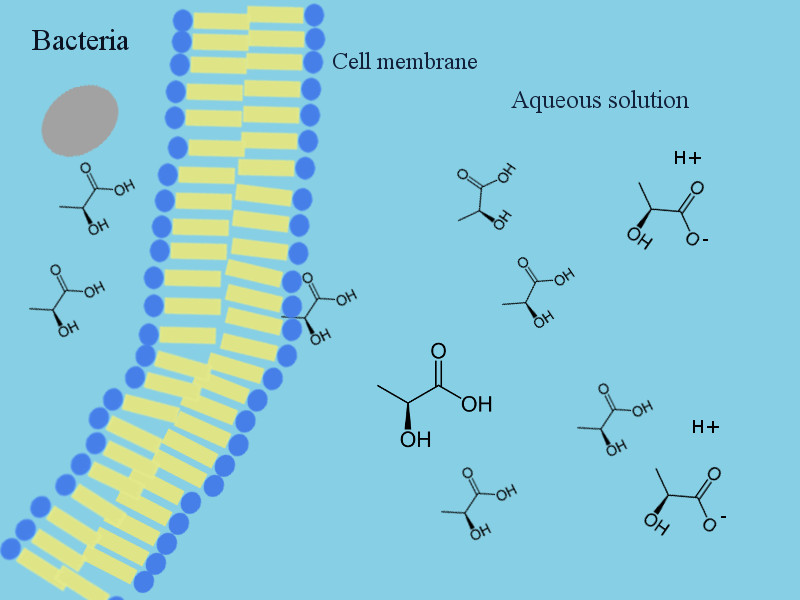
As earlier explaines, these weak organic acids are partially dissociated in water. It means at any given pH and depending on the pKa of that acid, a certain percentage of the acid is in acid form [HA] and a certain percentage is in salt form [A-].
Only the less polar acid form [HA] is able to penetrate the cell membrane and enter the inside of the micro-organisms. It means the [HA] can enter the cell whereas the [A-] has to remain outside.
This is why these ingredients are effective as undissociated acid, the salt can not penetrate the cell membrane
In other words, it means that where as sodium benzoate has a better solubility than benzoic acid, only benzoic acid can penetrate the cell membrane of bacteria and act as a preservative. When you purchase sodium benzoate as a stand-alone ingredient or in a preservative blend, you have to reduce the pH to increase its efficacy as a preservative. As sodium benzoate it's pretty much useless.
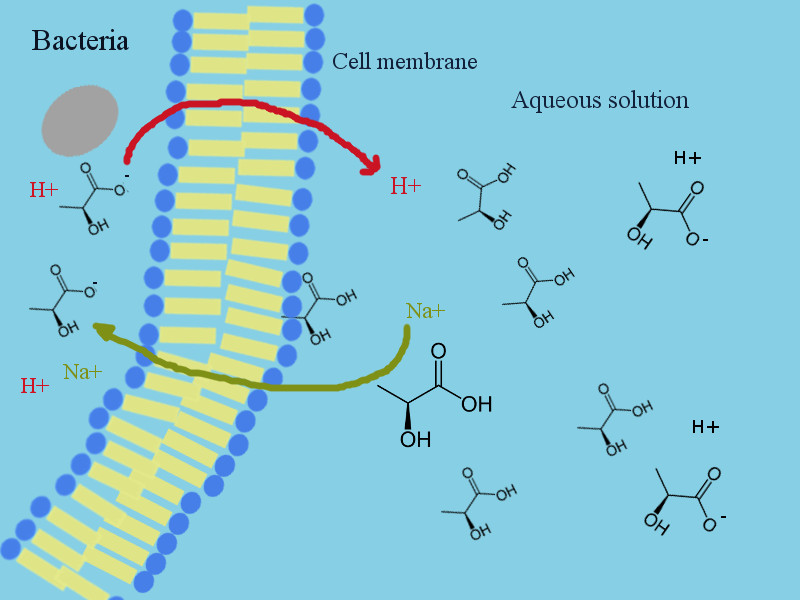
Well, the acids penetrate the membrane and enter the cell.
Inside the cell, they dissociate (remember the weak acids are partially dissociated to hydrogen cations and the residual anion). By degradation to [H+] and [A-] (in our example to hydronium cation and benzoate anion) they reduce the pH of inside of the cell. This pH reduction causes some instability and disorders in cell activities and enzyme functions. To balance this disorder, the cell sends the protonium cations out of the cell and exchanges them with sodium cations (electrostatic neutrality principle). This process is endothermic (needs energy). By sodium ions entering the inside of the cell, the osmotic pressure inside of the cell increases. The increase in osmotic pressure finally leads to cell disrupture.
Just to sum up the mode of action of "weak organic acid" preservatives:
- They destabilize micro-organisms by reducing the pH of the cells
- They destabilize micro-organisms by exchange of hydronium for sodium cations and increasing the osmotic pressure of the cells
- They destabilize micro-organisms by energy consumption (the ion exchange consumes energy)
For these weak acids:
- The acid form is the active form, the dissociated form is ineffective as preservative
- The concentration of the acid form depends on the pH, the higher the pH the lower the efficay
- Solubility depends on pH. Most of these acids are non-soluble or scarcely soluble at low pH
- To balance the solubility and efficacy the preservative is usually applied as a salt (more soluble form) and then is transformed to the acid form (the only effective form) by reducing the pH
- The solubility should be tested for each product/preservative at different pH and temperature ranges
This became a long post but I hope it can help you get the most of your preservatives and avoid wasting them and compromising the stability of your natural cosmetics.
Feel free to send us your comments and questions either per mail or posting them on our Facebook wall.
BeHappy and have fun