Sonntag, 5. August 2018
Why I love tocoperols and why you should love them as well
If you have been following my tutorials you know that tocopherol is one of my "must-have" ingredients and I can not stress enough how much I love it and how much I find it necessary.
To enlighten you about my crush on tocopherol I'm going to post a blog post about its amazing properties based on latest research results but before that I'm going to recap a blog post I wrote last year about vitamin E.
Consider this blog post as an introductory to vitamin E and tocopherols before going into details.

What is vitamin E?
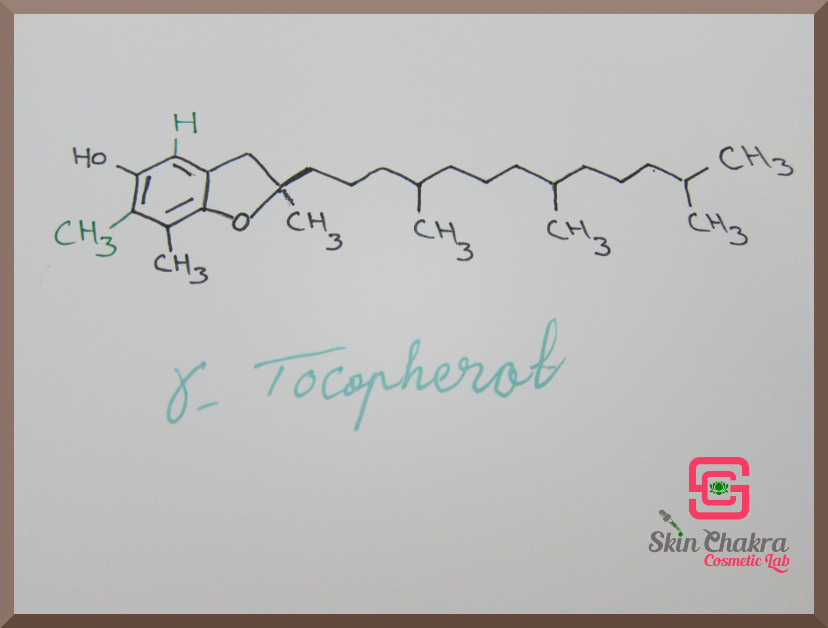
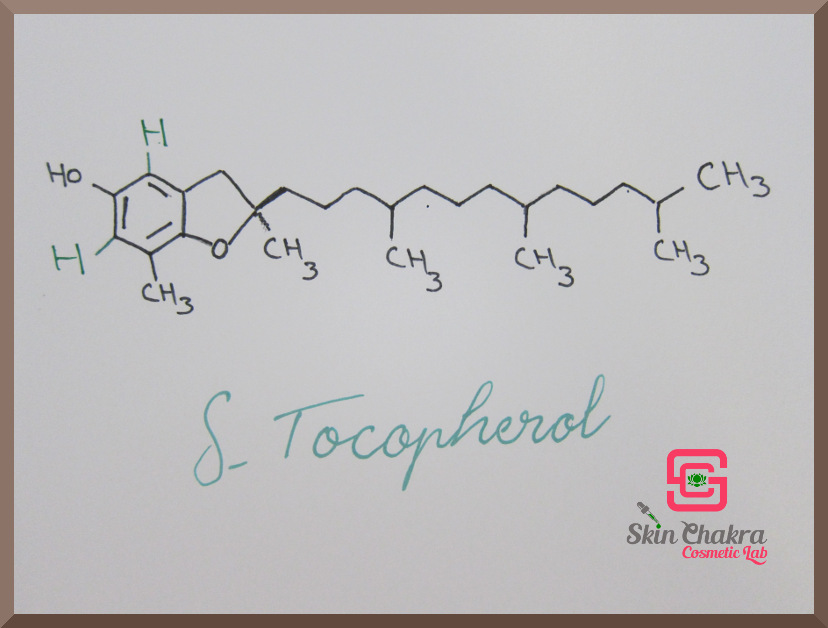
Vitamin E consists of 8 naturally occuring molecules: 4 tocopherols and 4 tocotrienols with slightly different chemical structure and activity. These are:
- alpha-, beta-, gamma- and delta tocopherol
- alpha-, beta-, gamma- and delta tocotrienol
these molecules are all lipophilic and have antioxidant properties.
Tocopherols are naturally found in seeds, grains nuts and related oils as well as in our body (in/on skin with alpha-tocopherol being the most abundant)
although alpha-tocopherol is the most prominent member of the family, all 8 molecules have antioxidant activities and are hence used in
- pharmaceuticals
- dietary supplements
- nutricosmetics
- cosmetics
- food
Before I go to the properties and applications, I want to bore you with the structure of tocopherols and tocotrienols. Tocotrienols are mainly used in pharmaceuticals and are not relative to our business. Just have a look at their molecule and their structural comparison to tocopherols and then keep it at the back of your mind that they are are vitamin E but not useful in cosmetics and reject the offer when somebody tries to sell you tocotrienols (I mean, if you're formulating skincare).
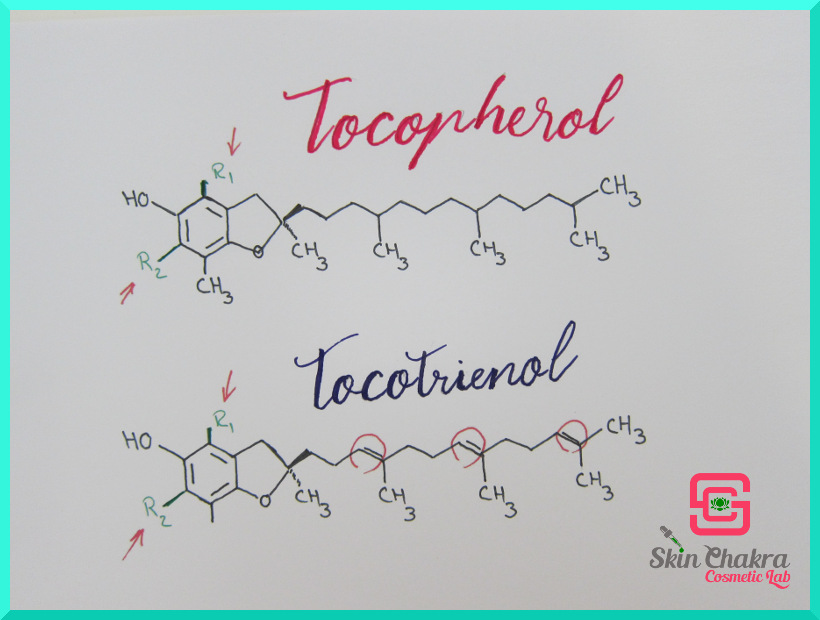
Looking at the above picture (with my scibbles) you see that the main difference between tocopherols and tocotrienols is in the hydrocarbon side chain. The chain is fully saturated in tocopherols where as it is polyunsaturated (three double bonds) in tocotrienols.
All the difference between tocopherols (alpha, beta, gamma and delta) is in the functional groups R1 and R2 (marked with red arrows).
Have a look at the following photos. The difference in the molecules is written with green ink.
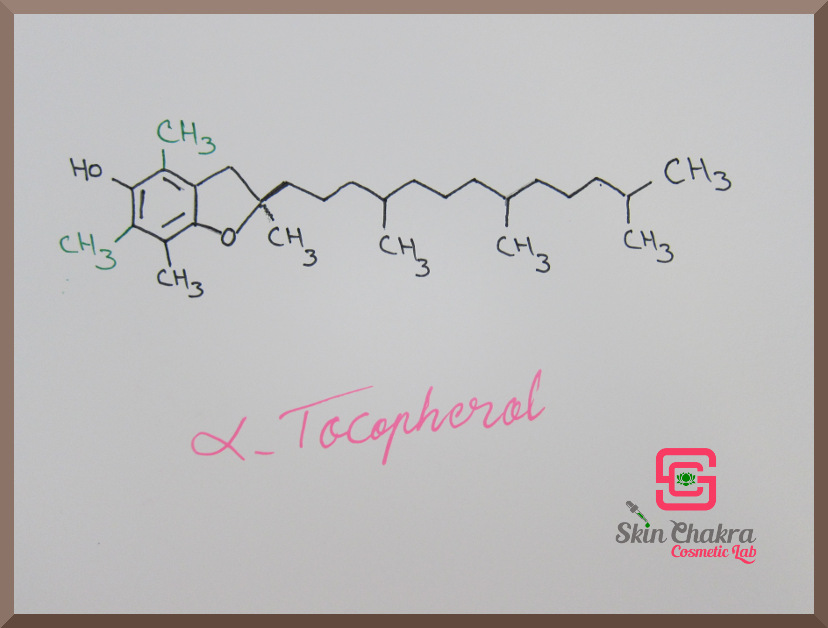
If you're not a chemist this doesn't have any real significance for you (it's like when I go to an art museum and try to find the difference between romanticism and impressionism) but I need to show you the difference anyway so that at least you know what it means when we talk about the 4 different tocopherols.
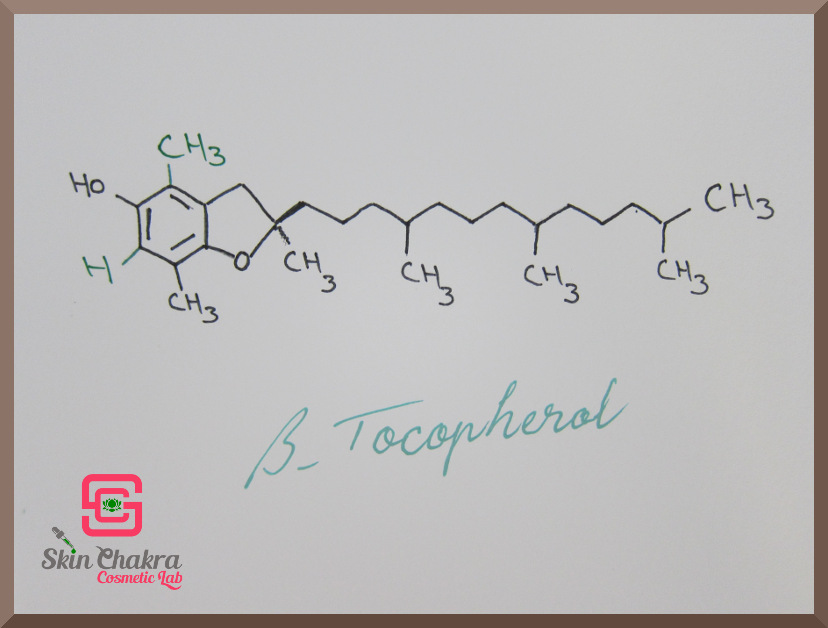
(believe me it doesn't have any significance to many chemists or wanna-be chemists as well). In plain English, you (and even all the chemists with a Nobel Prize in their drawer) wouldn't distinguish the difference by looking or sniffing or even applying the sample over their skin.
You may ask: why on earth shaould I bug you with chemistry of tocopherols when nobody can distinguish the difference? The answer is: because tocopherols are slightly different in their activities and biological properties.
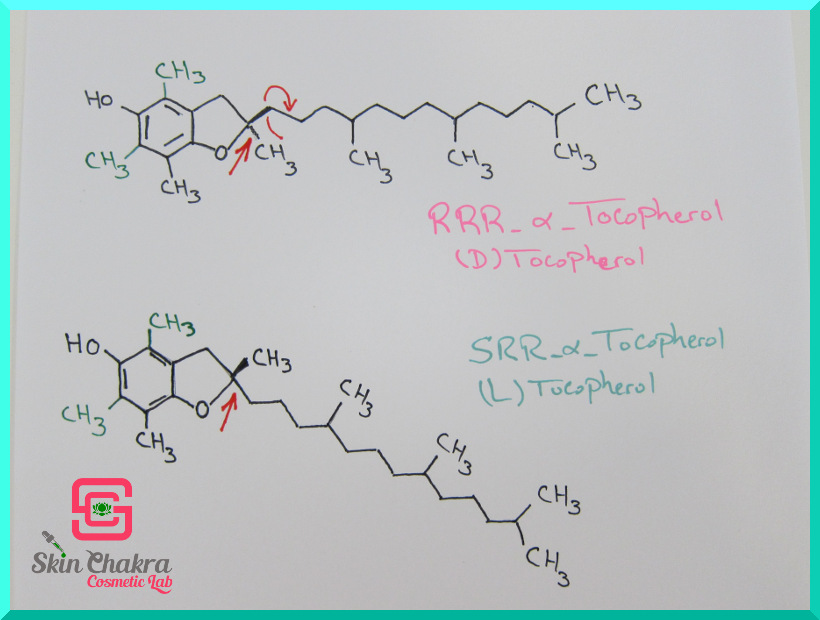
There is another difference which is not distinguishable even to Nobel Laureates but is extremely important to formulators and that is the stereoisomer (what on earth is an stereoisomer????).
Well, these molecules I have scribbled on paper are 3-dimensional in reality and can have different orientation in space. This is a specification of some molecules, especially natural molecules such as sugars, amino acids, vitamins and, as you'll see tocopherols and tocotrienols.
Molecules that only and only are different in their 3-dimensional orientation (if you draw them flat on a piece of paper they are exactly identical) are called stereoisomers.
In case of alpha-tocpherol, it seems as if somebody has screwed the hydrocarbon side chain 180 degrees around the x axis (may god bless the chemist who discovered stereoisomerism. I think it was van't Hoff but I'm not sure). The two different forms are called D- tocopherol and L-tocopherol respectively and there is only and only one single method to distinguish them (using polarized light).
Well you may ask: why on earth shall I be interested in this nonsense if the difference is not even distinguishable. Here is the answer:
Synthetic vs. Natural
Nature is very selective and creates only one form of tocopherol or tocotrienol and that's the D-form. Chemical synthesis however, is not that selective and usually creates equal amounts of the D and L form. This means, everything you find with the name DL-tocopherol (or tocotrienol) is synthetic or a mix of synthetic and natural.
Apart from the fact that depending on your business ethos and personal preferences you may want to avoid synthetic ingredients in your formulations, there is another significant difference between the syntheic and natural form:
Our body can only process the natural isomer of vitamin E. The synthetic form is biologically inactive.
This means you're wasting almost half of the content when you use synthetic tocopherols in your formulations.
Sources and available forms:
Natural tocoperols are mainly derived from plant seeds and oils (palm, soybean and sunflower oil are the main sources). Natural tocopherols are either offered as a mixture of alpha-, beta- gamma- and delta- tocoperols (only the effective D-isomer) or (rarely) as isolated alpha-tocopherol (this separation obviously increases the costs).
Synthetic tocopherols are usually offered as DL-tocopherol (with only half of the content being bioavailable)
All tocopherols (the D-form of alpha, beta, gamm and delta) are effective antioxidants. When it comes to protecting your ingredients against oxidation, gamma-tocopherol seems to be the most effective isomer. When it comes to biological activity and inhibiting membrane and extracellular lipid peroxidation, it seems that alpha-tocopherol is the most effective one.
IU vs gram and percentage
Most DIY-ers and skincare formulation students start with using vitamin E from food supplements and being confused when it comes to different units such as mg and IU. IU (international units) is mainly used in dietary supplements and food and has no use in cosmetic formulation. Apart from that, supplement tocopherols (even the natural ones) usually have additives such as sugar or emulsifiers which makes them useless in cosmetic formulation. Just in case you are interested in conversion and want to know how many mgs of vit E are hidden in your supplements:
1 IU of RRR-α-tocopherol (natural) = 0.67 mg of RRR-α-tocopherol (D-tocopherol)
1 IU of Racemic-α-tocopherol (synthetic) = 0.45 mg of RRR-α-tocopherol (D-tocopherol)
Available concentrations and forms
Tocopherols are available as powder (granules) or liquid with variable concentrations
Powders (granules) are usually used in the food and pharma industry and contain a substrate of starch or other material to help them easily disperse. These are not suitable for cosmetic applications.
Liquids:
Liquid tocopherol is available in concentrations between 10-70% in a carrier oil, usually sunflower or soybean oil or with an assay of about 95-98% in pure form (no carrier oil). The most common form is 70%. Although 90-95% tocopherol obviously contain more tocopherol and less soybean or sunflower oil, it has an extremely high viscosity and is very sticky and hard to work with, but if you prefer to have less carrier and more tocopherol, then go ahead and purchase the 90-95%.
No matter which liquid you choose, pay attention to the carrier oil and whether it is GMO or not.
Tocopherols in plant oils
Seeds and nuts (and related oils) contain various concentrations of different tocopherols and tocotrienols (D-alpha, D-beta, D-gamma and D-delta). These are present in native and unprocessed oils. Wheat germ oil for example contains around 2500 ppm total tocopherols and 1600 ppm alpha-tocopherol. Sacha inchi oil contains mainly gamma- and delta-tocopherol.
Refined and processed oils are usually stripped from their own tocopherols and trienols and then synthetic or natural tocopherols are added to them (not always but very often) as an antioxidant to prolong the shelf-life (don't ask me about the logic of this). Usually, when the plant oil is native and there is no additive, the INCI name doesn't mention any tocopherols (even if the concentrations are high). When tocopherols are added to the oil, the INCI name should contain tocopherols.
This is the same with essential oils and lipophilic plant extracts. If tocopherols (synthetic or natural) are added to the ingredient, the INCI name should declare this.
I hope you can digest this information till the next post when I'll share the reasons of my crush on tocopherols with you.
BeHappy and have fun