Sonntag, 12. Januar 2020
What the hell is "green chemistry"?
You may think I have lost my mind during the holidays. How on earth can chemistry be or become "green"?
In fact the public image of chemistry is as green as an elephant. Affected by "chemophobia" craze caused by pseudoscientific media, people throw the baby with the bathwater out and ignore all achievements of "chemistry" as a fundamental science and industry. "Nasty chemicals" and "chemical toxins" become the buzz words of the decade and "free from chemicals" becomes a marketing slogan (although highly illegal in most parts of the world).
A German chemist Karl Krauch complained several decades ago (even before chemophobia became a fashionable disease): "people died at the age of 30, nowadays their swear about chemistry in till the age of 90".
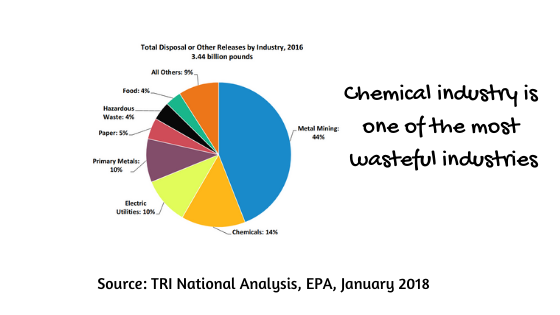
Chemical industry and chemistry as a science are indeed very messy and wasteful. Despite all the automation and progress in process control, synthesis, separation and analytical methods, there are huge amounts of waste, thousands of accidents and environmental damages caused by the chemical industry.
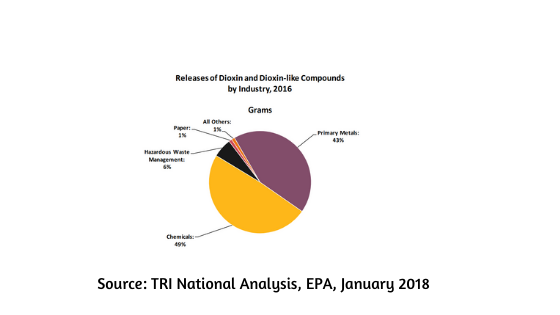
 Chemical industry is subdivided into different branches such as fine chemicals, petrochemicals, pharmaceuticals, detergent and cosmetics, etc. with some being more evil and wasteful than the others. To everybody's surprise, it's the pharmaceutical industry that creates the highest percentage of waste and not the petrochemical industry.
Chemical industry is subdivided into different branches such as fine chemicals, petrochemicals, pharmaceuticals, detergent and cosmetics, etc. with some being more evil and wasteful than the others. To everybody's surprise, it's the pharmaceutical industry that creates the highest percentage of waste and not the petrochemical industry.
"green chemistry" is a necessity to stop and reduce the damage (not even talking about reversing the damage already done) and not just "cool" or "fashionable" and it is a shame that even after 2 decades in the 21st century and after more than two decades from the first introduction of "green chemistry", it is not a mandatory discipline of chemical education and is still treated like an exotic branch.
History:
The first book proposing the concept and the pillars of "green chemistry" was published in 1998 by Paul T. Anastas (an EPA expert) and John C. Warner (from the university of Massachusetts). This book is still considered as the bible of "green chemistry" although it was the late Trevor Kletz who introduced the concept of "inherent safety" in the 80s of the last century. In his book about inherent safety he wrote more than 3 decades ago: "What you don't have, can't leak".
As you see, the concept is not quite new and in fact the ACS is hosting the 24th annual conference on green chemistry and engineering in this year but unfortunately the principles of "green chemistry" are not even mentioned during the undergraduate and graduate program in all universities (I don't have the exact statistics but I assume you can count the number of institutes implementing green chemistry on your fingers). Quite embarrasing, apparently there is no single university in Germany that has implemented the principles of green chemistry in its cirriculum (please correct me if i'm worong and if you are aware of any university in Germany that applies green chemistry in any of its under- or post graduate programs)
Chemistry is a strictly categorized discipline and for almost the last two centuries there have been organic chemists, inorganic chemists, physical chemists, theoretical chemists (who produce no waste and mess except on the paper), analytical chemists, and environmental chemists (I hope I haven't missed any class considering biochemistry and all its subdivisions are rather counted into biology rather than chemistry. I haven't mentioned polymer science but it is as messy and stinky and wasteful as organic chemistry). Every single group of the above chemists has done its own job with the organic chemists being the messiest of all and with one single aim: increasing the yield and reducing the costs . The rest was left to environmental chemists to clean up the mess and to toxicologists to calculate the risk by considering hazard and exposure.
I have been out of the university for more than 10 years now but as long as I can remember (and I think it has not changed in anyway if they don't implement the green chemistry principles) our laboratories were dangerous and stinky and wasteful. Dr. David Constable the director of the ACS GCI mentions: chemists use (waste) energy as if it were popcorn. and that is true. Not only energy but water, chemicals and other resources are used as if the literally grow on the tree.
(I come from the mouth pipetting and sulfochromic acid days. If a lab didn't stink to hell, it meant the students were not tedious and didn't work hard. To my shame, I can not even remember how we treated our waste during the undergaduate school, did we simply drain everything in the sink or did we collect them for disposal? I honetly can not remember)
In green chemistry, the boundaries between different disciplines are blurred. The organic chemist does not create something and then leave the mess to the environmental chemist to take care of. In green chemistry, the impact of the material and the process on the environment, human and resources should be considered even before you put your pen on the paper to design something.
Quite ironically, it's the industry that is the pioneer in green chemistry and not the educational section. This means, we educate conventional chemists with the mindset that resources last forever and grow on trees and then throw them into the industry that has finally realized there must be a change (either to please the public opinion and/or they have finally realized the whole process will be cheaper at the end). I have never understood politics and specially the educational policy. You can add this mystery to other policies nobody can explain and understand.
These are the 12 commandments of the green chemistry, they all seem quite obvious to us, but unfortunately they are not a matter-of-fact in the academic cycles and in the industry. Imagine that these principles ae not necessarily taught or even discussed in every chemistry cirriculum!!
1- Waste prevention
It is better to prevent waste than to treat or clean up waste after it has been created.
Chemistry is a messy science and industry (much worse in the academia than in the industry). In a traditional way of practicing chemistry (which is unfortunately practiced in universities and a great part of the industry), chemists and chemical engineers do their job which is the creation of a product and control of a process and then leave the mess to be processed by environmental scientists and other departments.
Huge amounts of waste are produced per each kg of product both in the lab and in the real world. Even if the waste is not harmful to human and the environment (which in most cases is), it should be somehow moved out of the way.
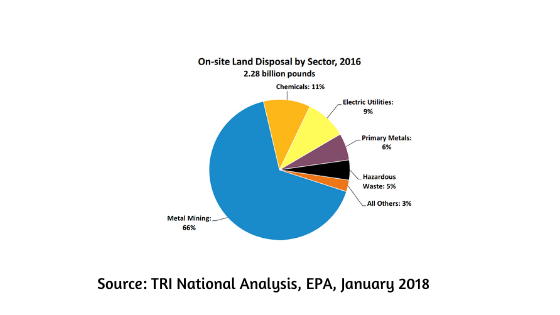 This can be land disposal, burning, recycling or doing nothing and hoping that the waste disappears on its own (as silly as it might sound, it is still practised).
This can be land disposal, burning, recycling or doing nothing and hoping that the waste disappears on its own (as silly as it might sound, it is still practised).
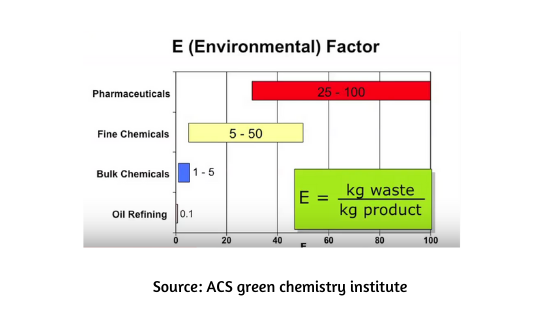 One of the important metrics of waste is the E-factor. It is the kg of waste produced per kg of product and guess which industry is the hero with the highest E-factor (thanks heaven it is not the cosmetic industry)
One of the important metrics of waste is the E-factor. It is the kg of waste produced per kg of product and guess which industry is the hero with the highest E-factor (thanks heaven it is not the cosmetic industry)
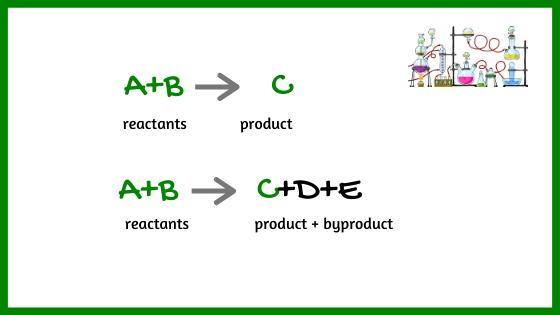
2- Atom economy
Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product.
This sounds as a matter of fact but it is not the way chemistry is usually done. Look at the following hypothetical reactions:
In the first reaction, all the atoms remain in the product. There is no waste. In the second reaction, A+B produce the same product C but together with D and E which are not the desired product.
3- Less hazardous chemical synthesis
Wherever practicable, synthetic methods should be designed to use and generate substances that possess little or no toxicity to human health and the environment.
This one sounds as well as a matter of fact to everybody who has not lost his wits but in reality (and certainly in the chemical lab and industry) this is not the case.
As Dr. D. Constable, the director of the ACS green chemistry institute says: It’s not that adhering to this principle is particularly difficult to do; it’s more that chemists are disinterested in doing it.
4- Designing safer chemicals
Chemical products should be designed to preserve efficacy of function while reducing toxicity
5- Safer solvents and auxiliaries
The use of auxiliary substances (e.g., solvents, separation agents, etc.) should be made unnecessary wherever possible and, innocuous when used.
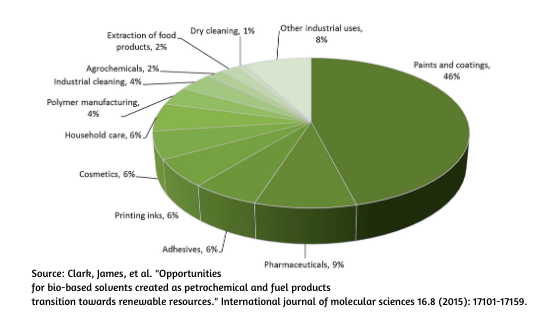
In a chemistry lab, solvents are used like fruit juice and the pharmacceutical industry has the highest solvent consumption after paint industry. Believe it or not, the solvents are burned and not recycled in the pharma-industry.
These are the solvents with the highest consumption volumes in the pharmaceutical (and partially cosmetic) industry (from the highest to the lowest volume):
IPA
Ethyl acetate
Methanol
Ethanol
Heptane
THF
Toluene
Acetic acid
Dichloromethane
Acetonitrile
(heaven save us!!). And we're still talking about green chemistry as an exotic branch in the 21st century!!
6- Design for energy efficiency
Energy requirements should be recognized for their environmental and economic impacts and should be minimized. Synthetic methods should be conducted at ambient temperature and pressure.
Waste of energy does not only happen in the "bad" chemical industry. Look at our own industry. Even the most "natural" ingredients are prepared under huge energy consumption conditions:
essential oils, plant extracts and hydrosols which are considered the purest (in terms of non-synthetic) ingredients, demand either heat or pressure or both of them. Time to rethink about the shades of green we're using in the cosmetic formulations.
7- Use of renewable feedstock
A raw material or feedstock should be renewable rather than depleting whenever technically and economically practicable.
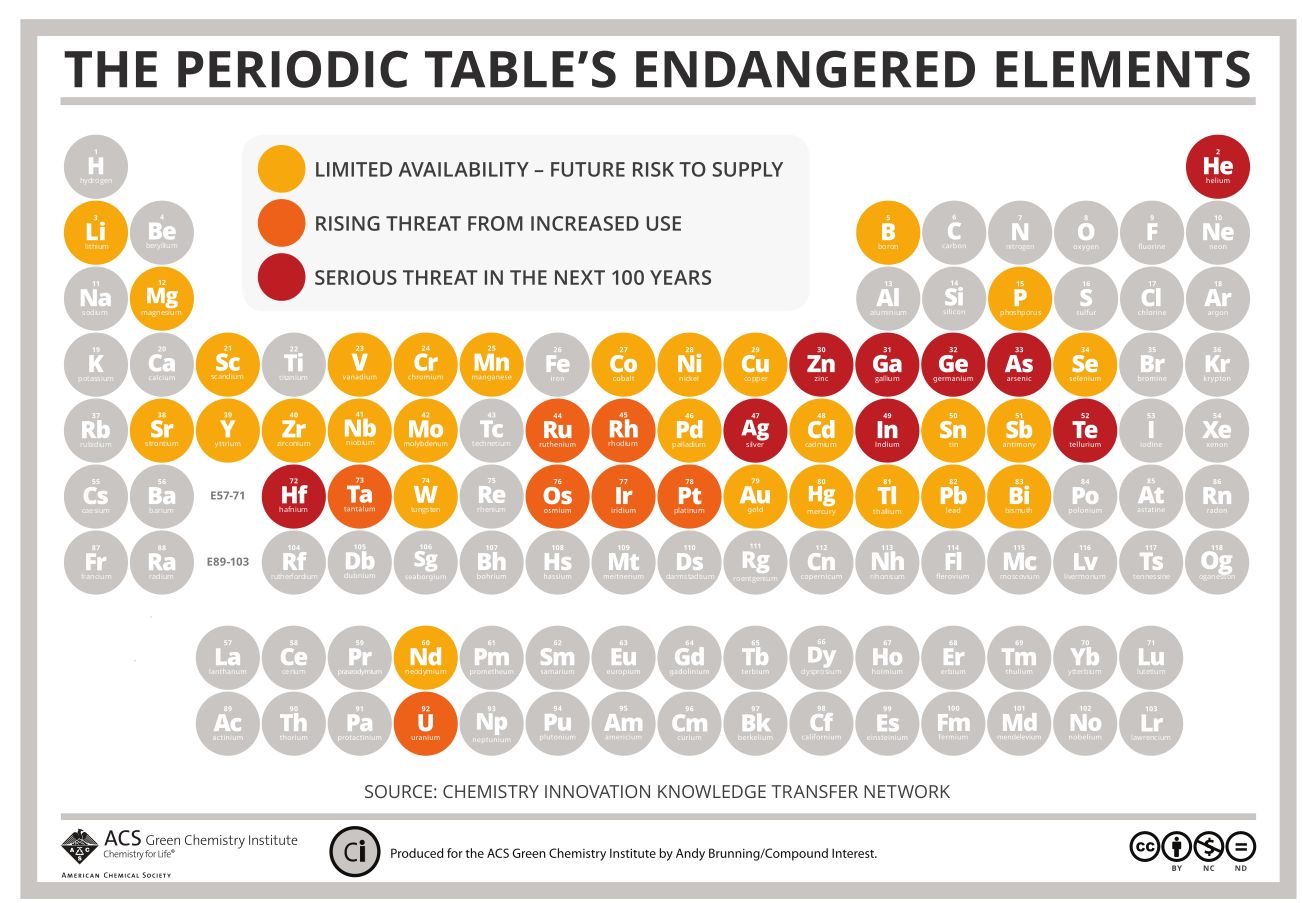
What is the price of our modern way of life? It is not only about fossil energy and extinction of living species, if we continue as we did the last 2 hundred years, we have to re-model the periodic table of the elements. Elements are simply disappearing and unless we change our methods or find another planet with these elements, the next generations will know these elements only from museums and history books.
8- Reduce derivatives
Unnecessary derivatization (use of blocking groups, protection/deprotection, temporary modification of physical/chemical processes) should be minimized or avoided if possible, because such steps require additional reagents and can generate waste.
If you are not a chemist, this principle probably does not tell you anything. Chemical reactions are very selective in nature. In the lab however, the reactions are neither really selective nor straightforward. In order to create/ synthesize a target molecule, some parts of the starting molecule are blocked, masked, derivatized in order to force a certain reaction and get to the target molecule. This is like hiding or protecting a part of the molecule and exposing another part to the reaction. Each derivatization, blocking or masking steps needs several additional steps and creates waste. Biomimetic and using enzymes is a "green" way of performing selective reactions without creating waste.
9- Catalysis
Catalytic reagents (as selective as possible) are superior to stoichiometric reagents
stoichiometric reagents are chemicals that participate in the chemical reaction. They transform the starting ingredients into the desired product but they are consumed and changed in this process. This means, at the end of the reaction, there is a waste that is not reusable and usually should be discarded.
A catalyst on the other hand, helps drive a reaction to the desired end point but is not consumed and transformed. This means it can be reused and reused. The industry is still using huge amounts of non-green catalysts (most of them are heavy metal derivatives and derivatived of the endangered elements) but the green chemistry is searching for safer and more sustainable alternatives.
10- Design for degradation
Chemical products should be designed so that at the end of their function they break down into innocuous degradation products and do not persist in the environment
Not only plastics and microplastics are non-biodegradable evils. Tons of chemical products (including many that are used in cosmetics, pharmaceuticals and detergent industry) are bioaccumulative and non-biodegradable.
In the conventional way of chemistry, the synthetic chemist creates an ingredient/ a product and leaves the job to other scientists to take care of the rest and calculate the bioaccumulation and environmental costs. In a green chemistry concept however, each and every single chemist shall consider the whole life cycle of the chemicals she/he is working with and the ones she/he is designing.
11- Real time analysis for pollution prevention
Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances.
Formation of possible hazardous by-products should be monitored and controlled during the process and not after the process is finished.
12- Inherent safer chemistry for accident prevention
Substances and the form of a substance used in a chemical process should be chosen to minimize the potential for chemical accidents, including releases, explosions, and fires
Each year, hundreds of work accident, explosions and unwanted release happens around the world (this includes explosions and release of toxic chemicals in the university labs) which cost huge sums of money, irreversible damages to the environment and unfortunately irreversible damage and even the life of people in and around the facility/accident scene.
In a traditional way of chemistry/toxicology
Risk= f [hazard*exposure]
this means, when working with hazardous material, we reduce the exposure and hence reduce/control the risk. Exposure reduction includes: wearing personal protection clothes, masks, goggles for the people working with those ingredients in the lab or the industrial scale. There are however cases when the exposure is not under control (leakage, accidents and explosions) and wearing protective masks and clothes is not effective. In such cases not only people working with those chemicals but even other people (and all innocent living species) near to the scene are endangered and suffer or loose their lives. Hundreds cases of petroleum leakege on the roads and in the sea and the tragic explosion of Bhopal (in 1987) are examples to prove that we need to reduce the hazard in order to control the risk.
Do you now agree with me that these principles should be a part of each chemistry cirriculum and not considered as an exotic branch of chemistry?
I think the 12 commandments of green chemistry should hang in a golden frame in each chemistry lab and that they have to make each chemist take the oath to the green chemistry principles the way the physicians take the Hippocratic oath.
For a pdf version of the 12 principles click here.
Further reading and references:
https://www.epa.gov/greenchemistry/presidential-green-chemistry-challenge-1998-academic-award-trost
https://www.epa.gov/sites/production/files/2018-01/documents/tri_national_analysis_2016_complete_0.pdf
https://www.mdpi.com/1422-0067/16/8/17101/htm
Green Chemistry: Theory and Practice
Green chemistry: an inclusive approach