Preparing stable gels and viscosity control are among the most significant challenges of each formulator. Viscosity control is most often performed with synthetic (e.g. polyacrylates) or natural polymers (e.g. Xanthan gum), each one having its significant characteristics, advantages and limitations.
One of the most versatile polymers in industry, cosmetics and pharmaceuticals and even in oral care products is a group of polyacrylates under the INCI name of Carbomer.
Carbomer family has several members with individual characteristics. All of them based on acrylate
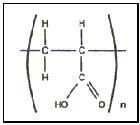
Each one having its specific characteristics concerning the viscosity, electrolyte tolerance, compatibility with other ingredients, etc. One of the most widely used Carbomers however is Carbomer 940 .
Carbomer is the main part of almost every cosmetics and pharmaceutical product which is offered as a gel, from cosmetic glitter gels to cuticle removers to gel toothpastes. It is a viscosity modifier, stabilizer, dispersant and emulsifier. Do you posses one of those small bottles of hand sanitizer gels in your purse to disinfect your hands as you're out of home? Have a look at the bottle. It contains about 0,5% Carbomer.

I've prepared hundreds of products based on Carbommer (have a look at my previous tutorial: Ice gel for heavy legs as an example). For the upcoming tutorials however, I want you to have a clear understanding of the chemistry and characteristics of the poylmer before you start working with it.
Disclaimer: Polyacrylates are synthetic polymers, if you generally avoid synthetic ingredients in your formulations, you do not need to read the whole story.
Carbomer 940 is a fluffy hygroscopic, light white powder which is dispersible, but not soluble in water and in alcohol.
As the polymer is dispersed in water or in alcohol, it has a pH of about 3 (very acidic) and it builds a turbid medium viscosity liquid (the molecules swell in water but not dissolve). This liquid is called mucillage.

Only after the acidic mucillage is neutralized with a base (such as sodium hydroxide solution, the same thing we apply in our lye preparation only at a much lower concentration, or triethanilamine) the polymer chains start to organize themselves
in an ordered manner building a clear, high viscosity gel

Since the gel has a high yield stress, you can disperse and suspend small particles (such as glitters in the above photo) or even air bubbles in it. Those gels are all prepared from a 0,5% mucillage.
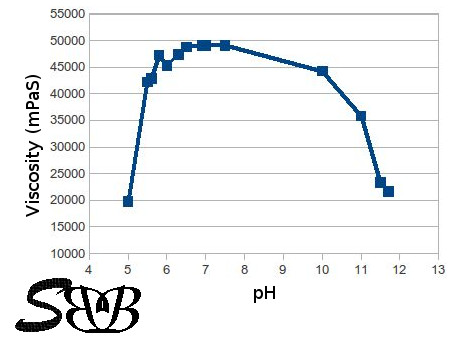
The viscosity (and the structure) depends on the pH. The highest pH (and transparency) is between 6-7. Over neutralization above a pH of 7 would cause a reduction in the viscosity and transparency of the product. This is something you should consider when choosing your preservative system. Those systems which are effective at a pH around 5 would not be suitable for Carbomer-based products. I'm applying Euxyl™ PE9010 which is based on Phenoxyethanol since years and I'm quite satisfied with it. If you decide to apply another preservative, make sure it is compatible with carbomer and your other ingredients.
In the below diagram you can see the viscosity of a 0,5% mucillage as a function of the pH
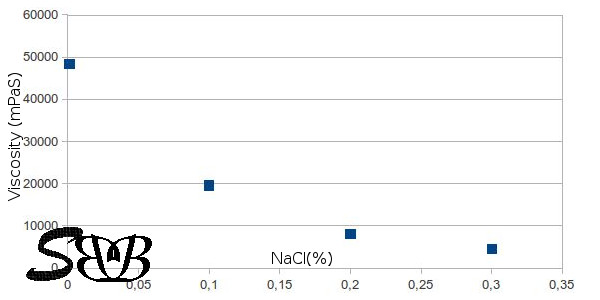
The viscosity is however very susceptible to electrolytes. There are other grades of carbomer with higher electrolyte tolerance but for Carbomer 940, the viscosity falls as soon as you have small amounts of any electrolyte in the medium.
In the following diagram you see the reduced viscosity as a function of added table salt (NaCl) to a 0,5% neutralized gel.
Working with carbomer is very exciting and fascinating. Preparing the mucillage is the most tricky part (but from my point of view it is easier than preparing Xanthan gum solutions).
Follow me to the next part for a tutorial on how to prepare Carbomer gels.
BeHappy and have fun

Swettis Beauty Blog am : Working with Carbomer (Part II)
skinchakra.eu am : PingBack
Swettis Beauty Blog am : Easymulsion- Macadamia oil cream gel