Sonntag, 17. Juli 2016
What happened to that beautiful colour?
If you have any experience in soap making then this is known to you that you can not have that beautiful red colour of red wine, berries or red cabbage in your soap. As soon as you add an extract, an infusion or red wine to the batter the beautiful red colour turns to an ugly greenish-brown colour.
This shocks very often the non-soap-makers who try to incorporate plant based colorants in a skin care or hair care formulation. They start with a beautiful and bright red and then are quite upset when their shampoo or shower gel turns to an ugly greenish-blue. At this stage most of them do not proceed with the rest of the ormulation and discard their product.
This has happened quite often during the last months since we're working on some surfactant projects and the students often come ask about the reason or want to go into the details of this colour change.
To explain the whole process, you have to refresh your chemistry background a little but (I know most of you hate chemistry and I'm very sorry to hear that. Just have a little bit patience with me, I don't go into details)
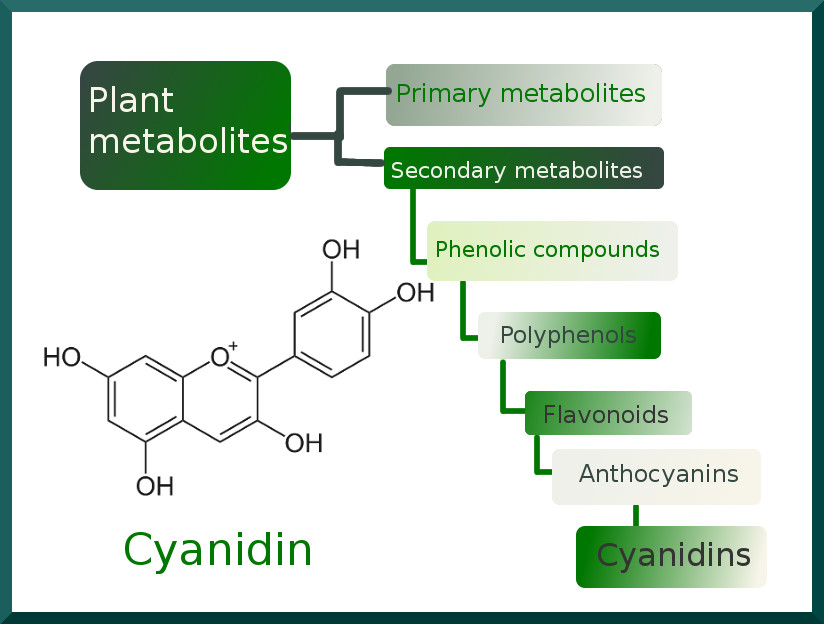
That beautiful red colour you see in red grapes, red wine, elder berry, red currant, red cabbage and many other herbs and flowers is generally called anthocyanidin. This is a very large group of plant metabolites.
Anthocyanins belong to flavonoids which is on its turn a subgroup of polyphenols. These are chemicals with different properties but their most important and widely studied property is its anti-oxidant power. This is why polyphenols and fruits/vegetables rich in polyphenols are so important in nutrition and in anti-aging skin care.
You may want to go back and read some of my older post about plant metabolites here and here.
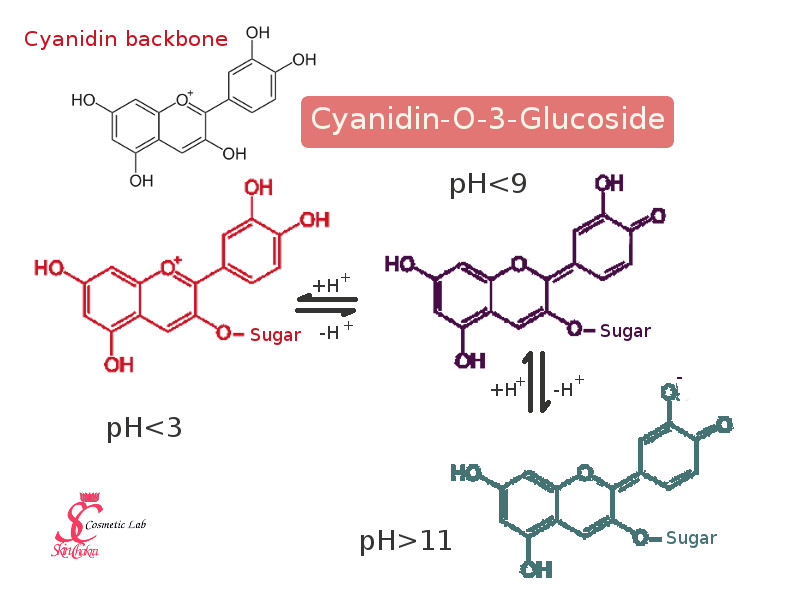
One of the most prominent molecules of the cyanidin group, and the main component responsible for the red colour of elderberry is a chemical class called "cyanidin-O-3Glucoside" (mercy. Don't worry you don't have to learn this by heart)
This creature (whose molecule might look like some strange aliens) is an aromatic molecule (can you remember benzene from your organic chemistry lessons?) and since it absorbs visible light at a certain wavelength it appears colourful.
By changes in pH this creature can turn to other creatures just by exchanging a hydronium ion (H+). As you can observe in the following diagram, the molecule has different forms at different pH ranges. These forms are very similar on the paper but in reality they absorb light at different wavelengths and hence appear to us in different colours.
Most of these colour change reactions are reversible. It means that when you increase the pH of an elderberry solution for instance from 3 to 12, the colour changes from red over purple to a greenish blue (or dirty gray in your formulation) but by reducing the pH the red colour is resumed. In fact some anthocyanidins are still used as pH indicators in school demonstrations. Red cabbage is one of the most widely used ones.
This is really helpful for skin and hair care formulations because most of our products have a pH of around 4.5-6.5. There are just some exceptional products that have a much lower or much higher pH (chemical peels, deodorants, cuticle remover or hair dyes are some examples).
Now this colour change very often happens when you add a surfactant to your formulation. Some surfactants, specially the APG group og surfactants are delivered with a high pH, mainly to protect them from microbial contamination. As you add decyl glucoside or lauryl gluoside or any other raw material with a high pH, you shift the pH of your product to alkaline. This is where you observe the sudden and frustrating colour change.
You know however that a shampoo or conditioner or shower gel or whatever skin and hair care product you have (except the product belongs to any of those exceptional categories named above) should have a final pH between 4.5-6.5 and you have to adjust the pH of your formulation by addition of an acid (citric acid or lactic acid for instance). In almost all cases, as you reduce the pH of the formulation the red colour is back (may be not as vibrant as the sole infusion but it is not greenish anymore).
If the colour comes from an extract or infusion that it not a main component of your formulation (used instead of water), you may even try to add the extract after you've reduced the pH to the neutral range. This will spare you the shock of the colour change.
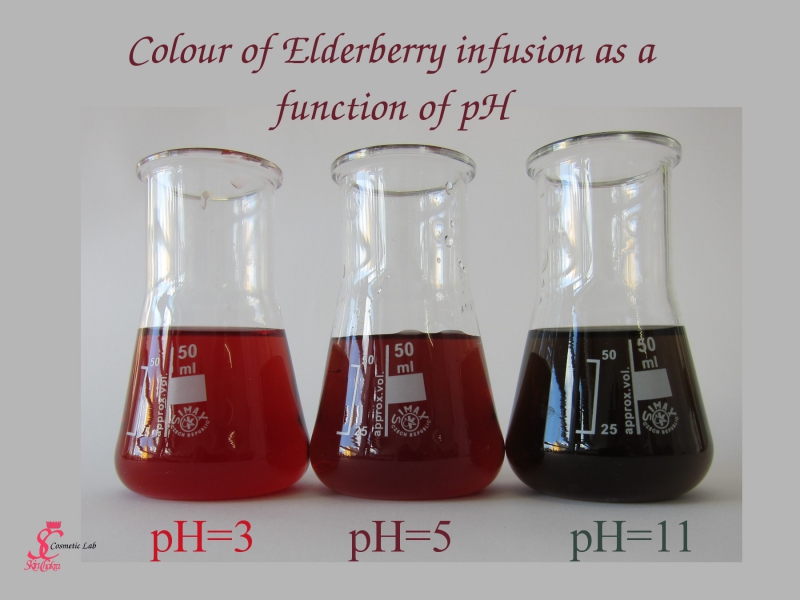
In the above photo we've varied the pH of an elderberry infusion from 3 (the left erlen meyer) to 8 (the right erlen meyer). The red colour becomes darker and darker.
Untill by a pH of around 11, the colour turns green.
So the next time you observe such colour change do not panic and do not discard your formulation, just reduce the pH of the formulation to the desired 4.5-6.5 and you'll have your pretty colour back.
Let me know if you have had such experience. You're very welcome to post your photos on our facebook page or tag skinchakra when you upload your photos on Instagram.