Donnerstag, 14. März 2019
How much is too much? Rancidity of plant oils
Tocopherols are one of my "must-have" ingredients and those of you who follow this blog and my formulations have certainly noticed that I add it to every formulation containing a lipid phase.
Please read our previous blog post about tocopherols before you proceed with this post.
Tocopherols (vitamin E) are naturally present in seeds and grains and as a consequence in many unrefined plant oils. If you are a subscriber to our newsletter, you have already received our e-booklet: Vitamin E content of plant oils as your subscription bonus.
 In refined oils (which is what the mainstream industry prefers), natural tocopherols and other unsaponifiables are separated from the oil and then they add 0,05-0,1% of a tocopherol or a blend of tocopherols (sometimes even a synthetic tocopherols) as an antioxidant to the oil (sounds crazy I know but that's the way the mainstream industry works).
In refined oils (which is what the mainstream industry prefers), natural tocopherols and other unsaponifiables are separated from the oil and then they add 0,05-0,1% of a tocopherol or a blend of tocopherols (sometimes even a synthetic tocopherols) as an antioxidant to the oil (sounds crazy I know but that's the way the mainstream industry works).
Tocopherols are not only added to the plant oils to prolong their shelf-life and inhibit their rancidity, they are added to finished products as well both to improve the oxidation stability of the product and to protect the skin lipids against oxidation. This is why vitamin E is considered an anti-aging ingredient and is added to almost all anti-aging formulations: It protects the skin lipids against oxidation, improves barrier function of the skin and accelerates wound healing.
Despite what many DIY-experts and raw material suppliers claim, vitamin E and tocopherols are no preservatives. They do not protect the ingredients or the product against microbial contamination. They are only and only antioxidants.
To understand vitamin E's chemical and biochemical function as an antioxidant, we first need to understand the oxidation process. (I try to keep it simple and don't bore you with the detailed chemistry)
Rancidity which mainly refers to edible oils is a collective term for the formation of unpleasant odours and deterioration of the fats and oils that makes them non pleasant (and sometimes unsafe). Although it is mainly referred to oils and fats, rancidity happens in the original nuts and seeds as well (have you ever accidentally bit into an old walnut, pistachio or almond?)
Rancidity does not only happen to edible oils and fats. Every triglyceride (oil, fat, wax, butter) could become rancid under certain circumstances (some are more susceptible to rancidity than others).
Rancidity basically happens via 3 pathways:
- microbial rancidity
- oxidative rancidity
- hydrothermal rancidity
At least one or all of these paths of deterioration can happen in a certain oil at different ratios and all of them lead to undesired by products with an off-odour (the typical rancid oil smell).
Let us separate microbial rancidity from the other two paths because it is not quite relevant to our cosmetic oils and butters.
We all know that contamination happens where and when water is present (water activity), This however, does not mean that there are absolutely no microorganisms present in a plant oil (plant oils and butters must have a low count of microorganisms but this doesn't mean they are free-from microorganisms). The beasts present in the oil can not grow and multiply and cause harm as long as there is no water present though. This means, if you purchase or prepare an oil under non-hygienic conditions (have you seen those oils they prepare at touristic sights? The way they prepare argan oil in front of your eyes in Moroccan bazars?), there are certainly a great number of nasties (microorganisms) in the oil. They can not cause any contamination as long as there is no water but as soon as you add water (in an emulsion for example) the nasties become active. Apart from contamination (which is not considered a rancidity), the enzymes in microorganisms help the microorganism digest the oil (the way our stomach enzymes break starch and fats and polysugars). During this digestion, triglycerides are broken to free fatty acids and glycerin. This kind of rancidity is very significant in the food industry when short chain free fatty acids (such as butyric acid) with the typical sour milk smell are released.
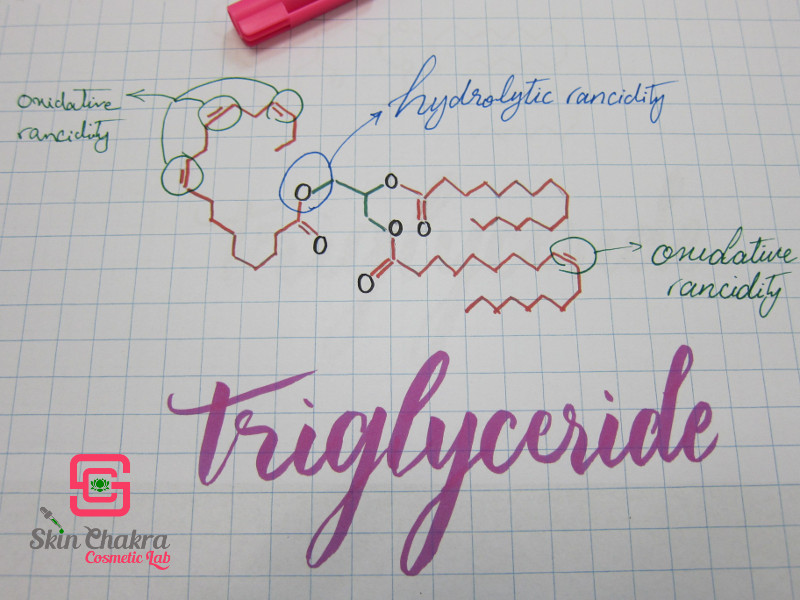
Fats, oils and butters consist of triglyceride blocks. Each triglyceride contains a glycerine block and 3 fatty acid blocks which may be identical or different.
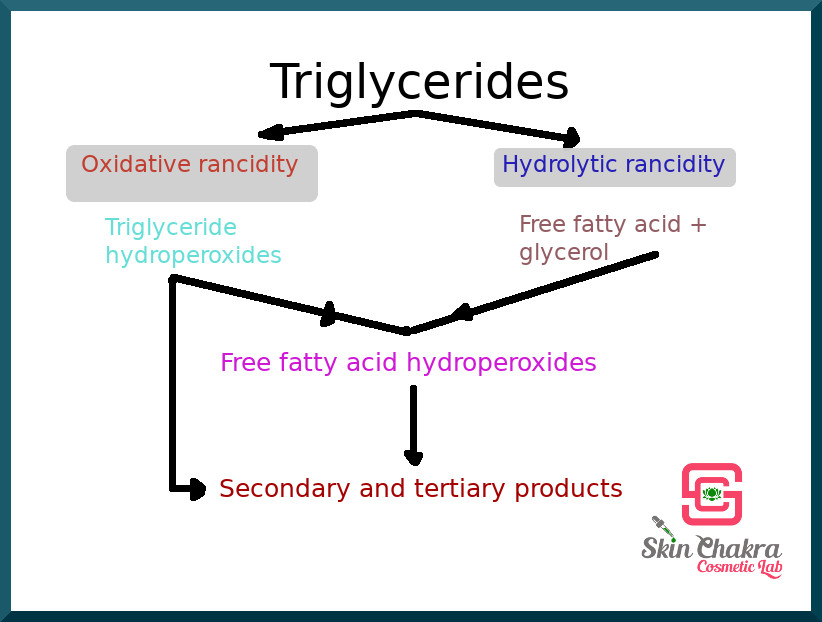
The hydrolytic rancidity affects the glycerine part of the triglyceride whereas oxidative rancidity affects the fatty acid part. This is where the iodine value (the degree of the unsaturation of the fatty acid) plays an important role. The higher the iodine value (the more unsaturation), the higher the susceptivity to rancidity and the higher the sensitivity to oxygen and light. This is the reason why you can heat some plant oils and should avoid heating some.
both pathways lead to the same end products via different pathways. The end result is the unpleasant odour, the sticky feel and the harmful peroxides.
Whereas hydrolytic rancidity is accelerated by water (this is why you should keep your oils under dry conditions) and heat, oxidative rancidity is accelerated by light, oxygen, free metals and heat.
 Antioxidants can inhibit (or slow down) the oxidative degradation of fats and oils (and finished products) and we'll come to that in the next part of this post (stay tuned for the next part)
Antioxidants can inhibit (or slow down) the oxidative degradation of fats and oils (and finished products) and we'll come to that in the next part of this post (stay tuned for the next part)