Last week, in part I of this blog post I explained:
- what are surfactants
- how do they behave in water
- what is HLB
- what makes a surfactant a emulsifier, a solubilizer and a detergent
In this part, I'm going to explain about the 4 main categories of surfactants. This seems to be a huge dilemma to most skincare formulation students without a background in chemistry.
For a detailed explanation of each category and its properties please go back to thi sblog post:
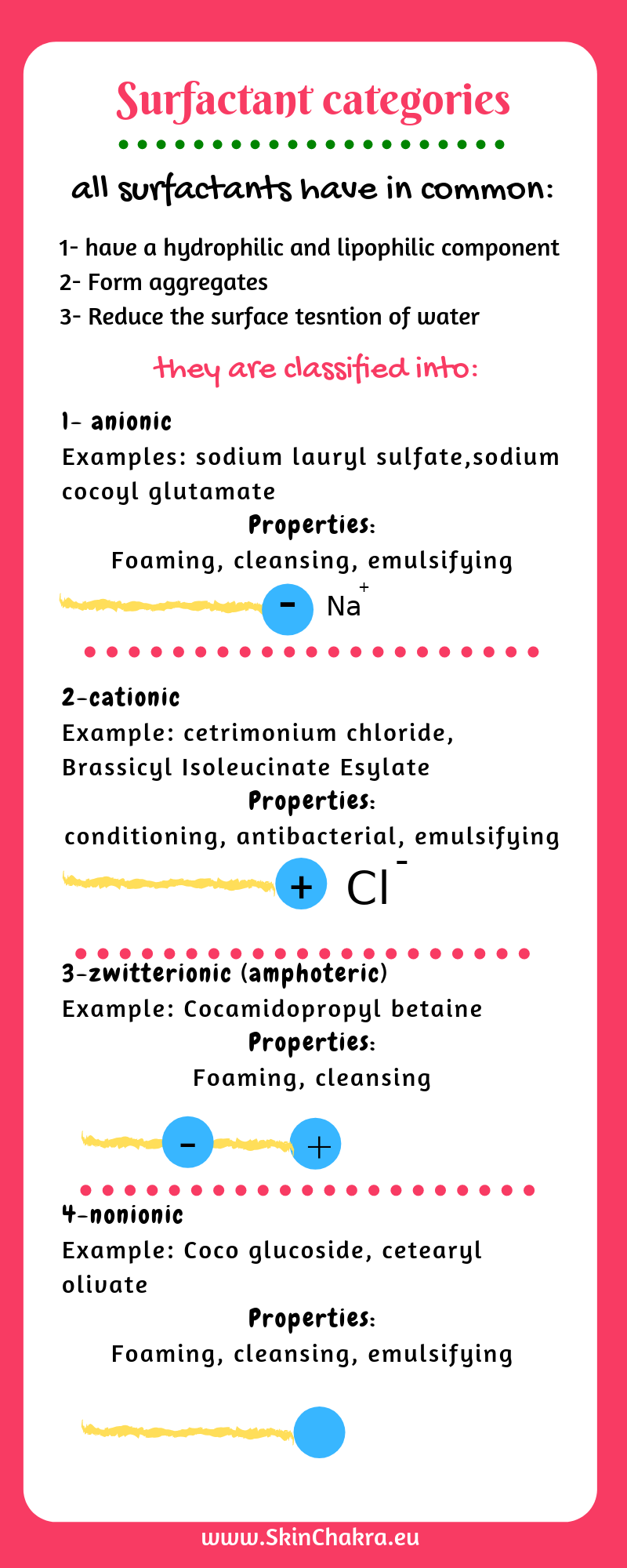
If you belong to the modern, smartphone generation who doesn't have the patience to read detailed texts, this infographic summarizes that long blog text:
There is one important fact that you need to keep in mind when you formulate with surfactants. No matter you make a hair conditioner, a facial cleanser, a shower gel or an emulsion serum. Compatibility of the surfactants you are using is the alpha and omega of formulation.
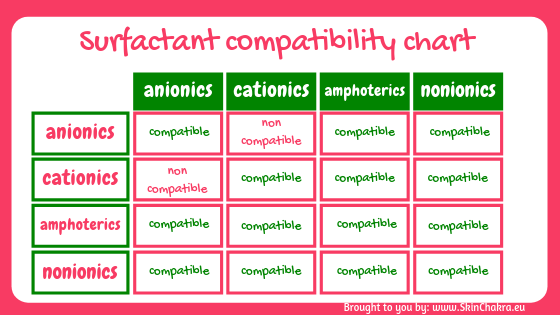
What happens when you blend an anionic and a cationic surfactant?
When an anionic and a cationic surfactant are blended, the long anionic tail and the long cationic tail build an insoluble salt that will separate from the solution as a sediment.
In case of classic surfactants this salt/sediment is easily observable. There are big chunks (like curdles cheese). In case of some modern surfactants, the sedimentation and the incompatibility is not that obvious. The inactive salt may even be water soluble but in any case you are inactivating both surfactants when you blend these categories.
Every rule has its exceptions:
indeed, there are a few exceptions to this rule, spcially in case of polymeric surfactants. Do not let yourself get confused or try to find out whether your anionic surfactant is compatible with cationics or not. Usually your supplier provides you with this information.
Just one more thing:
Surfactant active material
active material is the real concentration of the surfactant. This is very important when you formulate cleansing products such as shampoos, shower gels, facial cleansers etc. Consider you are using a surfactant with 30% active material. If you use 10% of this surfactant in your formulation, you're actually using 3% active material in your product. Now if you use a surfactant with 50% active material, you need only 6% of that surfactant to have 3% active material in your final formulation:
10*0,3 = 6*0,5= 3,0
Now to give you a rough approximation for formulating cleansing products, this is the guideline you can follow for your formulations.
Disclaimer: this has nothing to do with the legislation. This is a rough approximation I came to use during years of experience. You can prepare your products with a much lower or much higher active material. Keep in mind that active material has nothing to do with foaming. It is the scale for the real concentration of the surfactants. Your creation with 10% active of surfactant X might foam much less than your creation with 5% active of surfactant Y. (who has ever claimed surfactant chemistry is easy?)
1- Cleansing foam:1-5% active
2- Facial cleanser (cream cleanser, gel cleanser):3-10% active
3- Baby shampoo: 3-8% active
4- Shower gel, shower cream:10-20% active
5- Adult shampoo:
a) daily use, sensitive skin: 8-12% active
b) normal to greasy hair: 10-16% active
c) dry hair: 8-12%

What else?
as a comparison: the good old fashioned soap (saponified oils and fats) has an active between 75-100% (depending on the superfat content)
The syndet bars, shampoo and shower bars made with surfactants (no saponification involved) have an active between 20-60%.
I hope I could answer some of your questions about surfactants and you're ready to create some amazing products now.
BeHappy and have fun





