Donnerstag, 21. Juni 2018
Science made easy: Surfactants 101 (part 2)
In part I of this blog post we discussed about surfactants in general and their classification based on their ionic character.
Apart from the ionic character, there is another property that helps us classify surfactants and predict their behaviour, properties and applications.
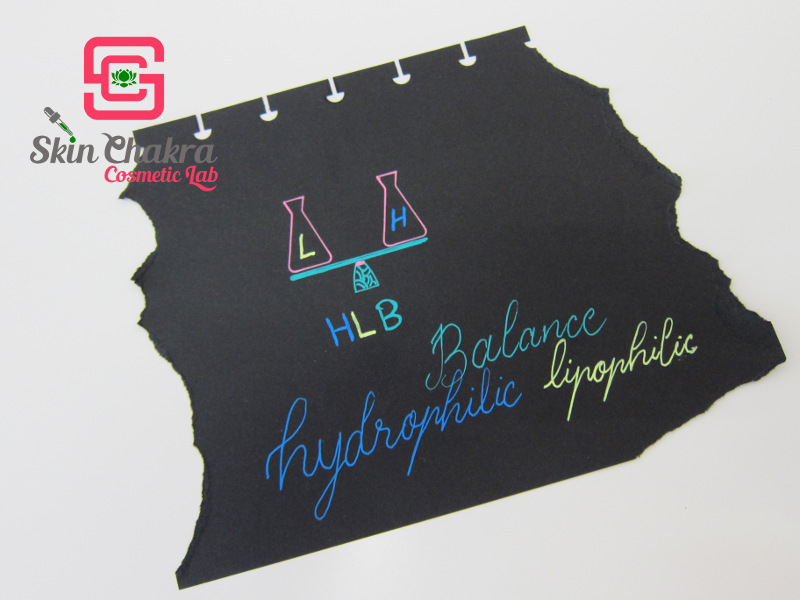
HLB (stands for Hydrophilic-Lipophilic Balance) is an old definition (almost as old as the term "surfactant" itself) that has been used to predict the properties and applications of surfactants. In "old-school" cosmetic chemistry and formulation educations it was taught very seriously and the HLB value of most surfactants were calculated up to the second decimal. Nowadays and with modern surfactants (specifically with PEG-free surfactants) the old HLB system is almost meaningless. In fact, for most surfactants the HLB value is not even mentioned or mentioned very broadly in a range on the product data sheet. In simple words, it is a numeric classification of surfactants defining the ratio between the hydrophobic and hydrophilic parts. The HLB varies between 0-18 with 0 being completely lipophilic and 18 completely hydrophilic.
As we've mentioned in the previous part, surfactants consist of a lipophilic part and a hydrophilic part. Now the balance between these two parts (HLB) determines the water solubility, properties and application of any given surfactant.
You don't need to go back and learn all about the old-fashioned and outdated HLB system. It is enough to be familiar with the term and be able to predict the properties and application of a surfactant (very broadly) by its HLB value
It is enough to know that, for example:
- a W/O emulsifier has an HLB between 4-6
- an O/W emulsifier has an HLB between 8-16
- a detergent (cleansing surfactant) has an HLB between 13-15 and
- a solubilizer has an HLB between 13-18
This is a proof why the myth and general misunderstanding: "that you can create both an O/W and a W/O emulsion from a single emulsifier by changing the blending order of oil and water phases" could not be correct.
Keep in mind that although emulsifiers, solubilizers and detergents are surfactants and have some common properties (solubilizers foam and detergents can solubilize essential oils or even emulsify low concentration of oils), they can not be interchanged.
Detergents (cleansing/foaming surfactants)
Among all surfactant classes, it seems that the detergents (those used in cleansing products) are the most mysterious ones and cause formulation students the most headache. This is why we're dedicating this blog post to this group of surfactants.
A surfactant must have an HLB between 13-15 (rather on the water soluble part of the spectrum) in order to be a detergent and cleanser.
This brings us back to another myth and general misunderstanding:
Some retailers claim (mistakenly) that coco- and decyl glucoside are oil soluble surfactants.
This couldn't be true because:
1- the HLB is in the water soluble range
2- Both of these surfactants are delivered as a ca. 50% aqueous solutions. They contain 50% water and could not be oil soluble.
Of course they both can dissolve some oil (it is their job), but this doesn't mean that they are oil soluble.
The following photo shows coco and decyl glucoside, 10% added to apricot kernel oil and blended with a magnetic stirrer for about an hour. As you can see and judge for yourself, none of them is really dissolved in the oil.
Apart from having an HLB value between 13-15, all detergents fall into 4 main categories regarding their ionic structure:
Based on the structure and ionic character of any given surfactant (detergent) you can estimate its properties and its application.
Disclaimer:
Before I proceed further, I need to explain that as to this time, the number of "natural" surfactants (detergents) are very limited. You can easily find hundreds of green and natural emulsifiers but when it comes to detergents, the industry is still in its baby shoes. The number of real "green" detergents are very limited. The mainstream industry is still stubbornly using PEG-derivatives and sulfates, two of the categories that are a no-go in a "green and natural" product concept. I have tried to gather surfactants that are basically acceptable in "natural" formulations but I'm afraid the choice is rather limited and miserable.
During the last couple of years, I made the painful (and expensive) experience that even some of the surfactants that are marketed as: "green, plant derived, sulfate-free, PEG-free and natural" contain additives that makes them useless for a true "natural" formulation. These additives are sometimes not even mentioned in the SDS (this is a shame) and only after several rounds of mail exchange with the supplier, they were revealed to us.
In this post, I will mention which surfactants COULD BE "natural" but please before making a decision to use them or not, make enough inquiries about ALL of the additives. Only after knowing about all of the additives, you can decide whether a given surfactant is suitable for "natural" formulations or not.
In case of any doubt, contact us per mail or one of our social media channels. We would be happy to answer your questions and make investigations.
Green or not green this is the question:
Except for soap nut, yucca extract and a few other saponin containing plant extracts, all other surfactants (including soap) are processed and not grown on a tree. Depending on the origin of raw material, the additives and the manufacturing process a surfactant could be acceptable in "natural" formulations or not. Apart from a few obvious cases such as PEG-derivatives and sulfates (which are generally not accepted), the surfactant might be fossil based, tallow based or plant based. Do not just focus on the name to decide whether you can use a surfactant in your "natural" formulations or not.
Read our blog post Can we use PEG-derivatives in "natural" cosmetics for more information
1- Anionic surfactants
As explained in part I of this blog post, the hydrophilic head of the surfactant carries a negative charge (an anion) which is then balanced with a cation. (Mercy on us, are you still following me?)
Anionic surfactants are among the most widely used surfactants when it comes to cleaning and foaming. Sulfates and sulfonates are the largest groups of anionic surfactants. You have certainly heard of SLS and SLES (sodium lauryl sulfate and sodium lauryl ether sulfate respectively). Have a look at the ingredients' list of mainstream shampoos and shower gels and SLES is usually the second ingredient after water (aqua).
Anionic surfactants generally have good cleansing and foaming properties and are hence used as the primary surfactant in cleansing products. Keep in mind that the better the cleansing properties the harsher the surfactant over skin (stripping skin lipids as well as external dirt). They are used in products such as shampoo, shower gel, liquid handwash, shaving foam, facial cleanser etc. This "good" cleansing properties has its own price. Sulfates, sulfonates and sulfoacetates (although this latest group is accepted in "natural" formulations) are quite sensitizing.
Let us have a look at some of the most common surfactants of this group:
Sulfates:
- Sodium (magnesium, potassium,ammonium) lauryl sulfate
- Sodium (magnesium, potassium,ammonium) coco sulfate
- Sodium (magnesium, potassium,ammonium) myristyl sulfate
Sulfates are basically harsh to skin and mucuous membrane and are generally not accepted in "natural" formulations. Even the sodium coco sulfate (despite the fact that it is derived from coconut or palm oil and not from fossil fuels) belongs to this group although most retailers try to disguise it as a "plant-derived" surfactant
Ether sulfates
- Sodium (magnesium, ammonium) lauryl ether sulfate (SLES for instance)
Ether sulfates are PEG-derivatives and are universally forbidden in "natural" formulations
Sulfonates:
- Sodium (magnesium, potassium,ammonium) dodecyl sulfonate
Sulfonates are hardly used in personal care. They are very common in industrial and institutional cleaning. Don't bother yourself with them. Just keep in mind that they are not accepted in "natural" formulations
Sulfosuccinates:
- Disodium (diammonium) lauryl sulfosuccinate
- Ammonium lauryl sulfosuccinate
- Disodium cocamide MEA-sulfosuccinate
Sulfosuccinates have good foaming properties and are generally considered as mild surfactants. Depending on the functional groups on the molecule and the origin of the starting material, they might be accepted or unaccpeted in "natural" formulations
Ether sulfosuccinates:
- Disodium laureth-2 sulfosuccinate
- Disodium cocamido PEG-3 sulfosuccinate
These are PEG-derivatives and are universally not accepted in "natural" formulations.
Isethionates, glutamates, sarcosinates, taurates
- Disodium/sodium cocoyl glutamate
- Potassium cocoyl glutamate
- Sodium cocoyl sarcosinate
- Potassium cocoyl taurate
- Sodium lauroyl sarcosinate
These functional groups belong to mild anionic surfactants with moderate cleansing and foaming properties and much less sensitizing than sulfates and sulfonates. Depending on the origin of the starting material and functional groups on the molecules, these could be accepted or non-accepted in "natural" formulations.
2- Cationic surfactants
In cationic surfactants, the hydrophilic head of the surfactant carries a positive charge (a cation) which is balanced with an anion (usually chloride, bromide, methosulfate)
Cationic surfactants have a very limited application mainly as conditioning (hair and fabric) and anti-frizz and styling agents.
Most cationic surfactants are derivatives of "trialkyl ammonium", generally called as "quats". This functional group is not quite accpeted in sustainable and "natural" formulations because of its bioaccumulation and environmental aspects.
At this point, Brassicyl isoleucinate esylate is the only non-quaternary cationic surfactant which is accepted in "natural" formulations.
Keep in mind that cationic and anionic surfactants are not compatible by nature. If you plan to create a cleansing conditioner, a co-wash or a conditioning shampoo, you can not blend a cationic and an anionic surfactant in your formulation. In this case, you need to avoid anionic surfactants and use amphotheric and anionic surfactants
3- Nonionic surfactants
These surfactants do not carry any charge and are hence compatible with all other surfactant groups. Nonionic surfactants are usually used as complementary or secondary surfactants. They usually do not foam much as standalone surfactants but can enhance the foam volume and durability in synergystic blends with anionic and amphoteric surfactants. Apart from foam boosting they can enhance the consistency, viscosity and texture of the formulation.
Most prominent "natural" representatives of this surfactant group are the
alkyl poly glucosides:
- coco glucoside
- decyl glucoside
- lauryl glucoside
if you're working on a palm-oil-free concept, inquire about the origin of the surfactant. Although the glucoside part of the surfactant is usually derived from corn, potato or sugar, the alkyl part is most often palm-derived
ethanolamide derivatives:
Have a look at the ingredients' list of mainstream shampoos and shower gels. At least one in every three product contains a DEA or MIPA derivative (cocamide DEA, lauramide MIPA for instance). These are universally not accepted in "natural" formulations (don't get mislead by the coco part) and it is a shame they are still allowed to be used in personal care products in most parts of the globe (including the EU).
4- Amphoteric surfactants
Amphoteric surfactants belong to the mildest surfactant groups. They are usually very gentle to skin, hair and mucuous membrane and improve foam volume and durability in combination with anionic and nonionic surfactants.
Theoretically, amphoteric surfactants carry both anionic and cationic charge on the same moleule (this is why they are called zwitterionic). In the common pH range for personal care products (4,5-6) they act something between both anionic and cationic surfactants: foaming, cleansing, detangling, conditioning. Amphoteric surfactants are compatible with all other surfactant classes.
Unfortunately, there are not much "natural" amphoteric surfactants available on the market and:
- Betaines (cocamidopropyl betaine)
- cocoamphoacetates (sodium cocoamphodiacetate)
are among the rare representatives of "natural" amphoteric surfactants.
Blending surfactants
You hardly see a professional product using one single surfactant. Surfactants are usually blend to:
- reduce the sensitization and enhance mildness
- improve foam volume, texture and stability
- enhance viscosity and texture of the product
- improve stability
Don't be afraid of failures and ugly blends. In worst case, you can use your blends as bath cleaners (shall I share a photo of our ugly and unlucky preparations that we use as toilet cleaner?)
Blend your surfactants to prove yourself as an experienced and talented formulator. Keep in mind that higher concentration does not necessarily mean higher foam or viscosity. You may reduce the foam or viscosity by overdosing a surfactant in a blend (I know you'll love me for making your life as a formulator so easy)
Everything at a glance
| Surfactant type | Properties | Applications | Compatibility |
| Anionic |
good cleansing and foaming properties Usually primary surfactant |
all cleansing & foaming products: shampoo, shower gel, micellar water, bath foam, shaving cream etc. | not compatible with cationics |
| Cationic | conditioning, anti-frizz. No foaming or cleansing | hair conditioner, co-wash, cleansing conditioner, conditioning shampoo, conditioning balm, anti-frizz and detanglers | not compatible with anionics |
| Nonionic |
moderate to good foam, viscosity enhancer usually secondary surfactant |
almost all products from shampoo to conditioner and everything in between | compatible with all other surfactants |
| Amphoteric |
foam enhancer secondary surfactants |
all cleansing and foaming products, conditioning and anti-frizz & detanglers | compatible with all other surfactants |
This was a very dry stuff and I know you're not going to love me for this long blog post. Feel free to shoot your questions, and critics.
BeHappy and have (a little bit) fun