For part I of this article please click here.

In part I we discussed Lipids and we came to the attractive subgroup of Triacylgycerides and waxes.
Let's exclude essential oils and fragrance oils from our discussion forever.
Fragrance oils may have a synthetic nature, partly synthetic or a natural nature. It means they are a combination of hundreds of individual ingredients, most often diluted with a solvent. They are oil soluble (lipophile and hydrophobe) but even those with natural origin are not lipids and therefore are not real oils.
Essential oils are mainly distilled (like Rosemary oil) or expressed (like Grapefruit oil) from different parts of plants (bark, peels, leaves, flowers etc.). They are oil soluble (lipophile), natural volatile materials. But they are not as well considered as oils or lipids.
These are very broad and simple definitions of FOs and EOs and we don't want to go to details now. It is only to specify that they are not considered lipids or oils.
Let's separate waxes as well for the moment and concentrate on Triacylglycerides (a relatively modern name for triglycerides).
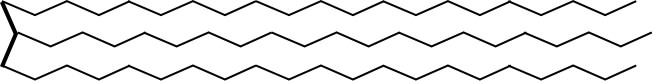
This is a simplified Triacylglyceride molecule.
At the right hand of the molecule, you observe three long hydrocarbon chains. These may be or may be not identical in structure and length. At the left hand you see a glycerin molecule. Three hydrocarbon chains are all connected to a single glycerin molecule via an ESTER-bound: (Triglyceride or Triacylglyceride).
This is a more detailed structural picture of Triacylglycerides:
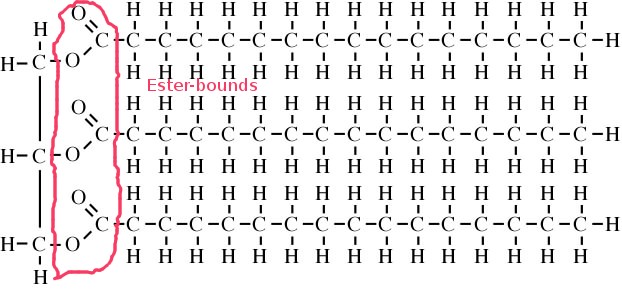
The left hand side shows a glycerin molecule. The part marked with red is the ester bound and in the right hand side there are three hydrocarbon chains which are bound together.
This is the simplified common structure for all that we call Butters, Oils or Fats. From lard and goose fat over cacao and shea butter to avocado oil, they all have the same skeletal structure.
Isn't that amazing?

Now we come to the difference:
Fats have a titer point higher than 40.5o C. They are solid @room temperature. Oils have a titer point below 20o C. They are liquid @room temperature. Butters have a titer point above 20o C and below 40.5o C.
(In our daily communication, we talk about melting of wax, or melting a soap base, melting lard, candle etc.. From a physical point of view however the definition "melting point" is applicable only to pure materials, let's say pure Iodine for example. For mixtures, specially for fats and in the petroleum industry the term "titer point" is a more common definition.) For a better understanding of titer point and melting point click here.
Look at the following ASTM methods for determination of Titer point in fatty acids: ASTM D1982 - 85 and ASTM D5565.
Don't miss part III of this article. We'll discuss about the structural differences of fats, oils and butter.

Swettis Beauty Blog am : Butters, Fats, Oils and Co. (part I)
Swettis Beauty Blog am : Butters, Fats, Oils and Co. (part III)