
For part II of this post click here.
In the previous part we realized that the main difference between fats, oils and butter lies on the titer point.
Fats, oils and butters are all Triacylglycerides which belong to Lipid-Family.
But what is the origin of the difference in titer point?
We've seen that Triacylglycerides are made from three hydrocarbon chains and one glycerin molecule connected via an ester bond together. In fact, regarding the below diagram you notice three fatty acids on the right hand side and one glycerin in the left hand side.
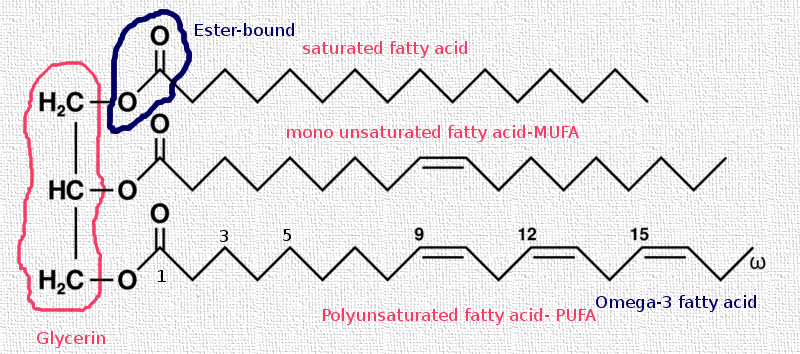
As you observe in the above sketch, you realize that it is the right hand side of the Triacylglyceride molecule which makes the whole difference, the left hand side, the glycerin is common in all fat and oil molecules.
It is the fatty acid which dictates the titer point, the spreadability, the emmoliency, the feel, the drying and the shelf life of a given fat or oil and even a finished product.
What are fatty acids?
The simplest organic acid is called formic acid with a molecular formula of HCOOH.
The second one, acetic acid or our table vinegar has one Carbon more with a formula as: CH3COOH. You may create bigger and heavier organic acids by adding one CH2 group to the basic molecule (HCOOH). This is called a homologous sery which is growing with adding CH2 to the basic skeletal structure.
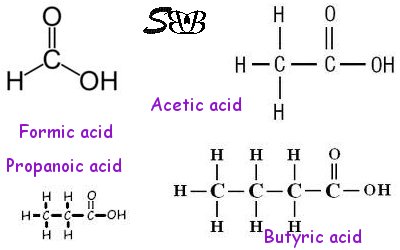
These mentioned molecules are not of our interest. They are short chain fatty acids (SCFA).
Plant extracted oils and fats have carbon atoms from C6 (Caproic acid) to C34 (Geddic acid). However the most part of the distribution remains between C16-C18.
It is interesting to know that nature only creates fatty acids with even number of carbon atoms: C12, C14, C16 etc. Fatty acids created in the laboratory contain both even and odd numbered carbon atoms, C12, C13, C14, C15 etc.
It is the chain length (Carbon atom numbers), the nature of the C-C bond (single bond or double) and the location of the double bonds which dictate the main characteristic of a fatty acid and of the oils and fats containing them. A double bond is the same as an unsaturated bond, what we know as unsaturated fatty acid from food industry for example.

The longer the carbon atom chain, the heavier the molecule. It means fatty acids with higher carbon atoms have a higher titer point and are semisolid to solid @ room temperature.
The more unsaturated bonds in a given chain, the more susceptible the oil to oxidation (higher Iodine value).
A fat or oil is a complex combination of 10s of fatty acids and is a mathematical average of the contributing fatty acids.
Follow this discussion to part IV.

Swettis Beauty Blog am : Butters, Fats, Oils and Co. (part II)
Swettis Beauty Blog am : Butters, Fats, Oils and Co. (part IV)