Donnerstag, 25. Mai 2017
How to adjust the pH of your cosmetic products
pH measurement is one of the most basic steps in creating skin care (and in every chemistry lab) yet is could be quite intimidating if you can not remember the basic from your highschool (or college) days or if you've deviated yourself during chemistry sessions (like many chemistry haters have done).
It is not only mandatory that the pH of the cosmetic products remain in a certain range to guarantee consumers' safety, it is essential in product stability, and most importantly, in natural cosmetics it is essential for the performance of the preservative system you are using.
Let's sum up why we need to meausure and adjust the pH before we go into details:
- The pH of cosmetic products designed for everyday application must be compatible with the physiological skin pH (which is 4,5-5,5).
One exception is the good old soap (Fatty acid or triglyceride bases soap. The one you make by saponifying oils with lye and letting it to cure) which by nature has a much higher pH (around 9-10). This makes the natural soap a little bit controversial. Soap lovers and admirers use it despite the fact that it kicks the pH of their skin out of balance. However, a young and healthy skin regains its physiological pH after a while. Some don't use soap on their facial skin and others use a toner after applying soap to bring the pH back to its physiological pH. It is personal preference and it is out of the scope of our post.
Commercial soaps claiming a neutral and balanced pH are no real soap. They are not made by saponification of a fat or an oil. They are blends of surfactants pressed together as a soap bar (or made as a liquid handwash)
- There are certain products with a much higher or much lower pH than the physiological range (4,5-5,5). These include chemical peels, hair dyes and hair strengthening products, chemical hair removal products. These are however, no everyday use products and are applied only occasionally and sometimes by a trained hair stylist or aesthetician. Even for these products, the pH should be measured and adjusted to guarantee their stability, safety and performance
- The pH of cosmetic products should remain in a certain range so that the colour, viscosity, skin feel and physicochemical stability remains intact during its shelf-life and during its application by the consumer
- Measuring pH, in not only an important step in manufacturing cosmetics, it is quite important is stability testing of your product. If the pH of a shampoo or a lotion changes after 3 or 6 months of storage, you should be alarmed about the stability of some of your ingredients and the overall product.
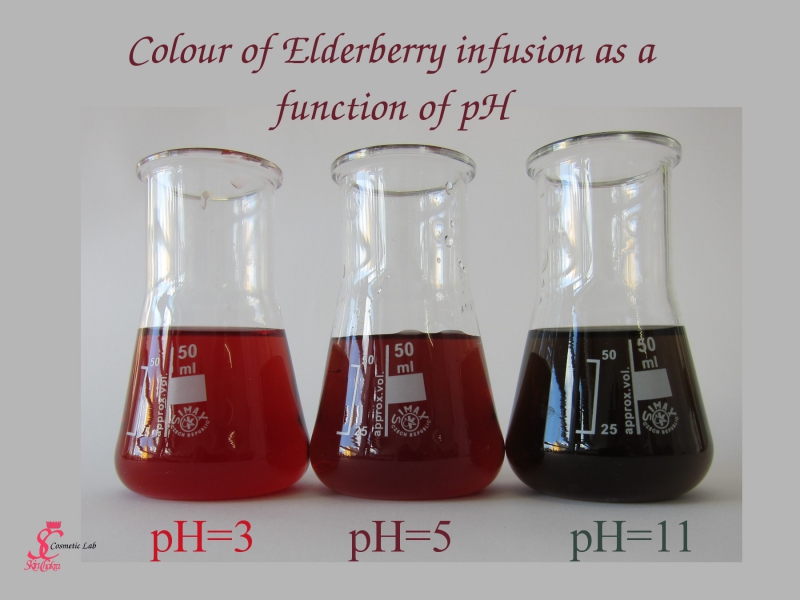
- Specially in "natural" hair and skin care, when we avoid synthetic colourants and rely on plant oils and herbal extracts to show their beautiful colour, the colour is extremely dependant on pH and you need to keep the pH in a narrow range to avoid changing the colour of the product
Read our previous post: What happened to that beautiful colour?
- Most importantly: almost all of the preservatives we use in natural hair and skin care are weak organic acids or a salt of an acid (benzoic acid, potassium sorbate, levulinic acid, salicylic acid). For these weak organic acids, the solubility and the performance is extremely pH-dependant. It means, if you don't keep the pH in a certain range, either your preservative will become insoluble and fall out of the solution (it becomes ineffective and it affects the physical stability of the product) or it becomes less effective or completely non-effective depending on the pH.
Read our previous post: How to protect your "natural" preservatives from deactivation
Now let's come to the practical part which always is a huge monster to new students of hair and skin care formulation.
pH measurement:
1- pH is an expression of the hydronium ions (H+, or H3O+ to be quite exact) in water. Where there is no water, there is no hydronium ions.
It means, in clear text that:
You can not measure the pH of a nonaqueous product such as an oil blend or a balm
This is one of the most frequently repeated mistakes skincare formulation students commit at the beginning. Even if you disperse your balm or oil in water, it is the pH of the water you are using and not the pH of the product.
pH has absolutely no significane and meaning where there is no water
2- Usually, the product (or the raw material) is diluted in distilled water. For raw matrial, you can find the dilution in COA (certificate of analysis) or SDS (safety data sheet). Very often, it is a 10% dilution. In some cases it might be 5% and for some exceptional cases like hydrosols, the pH is measured directly.
When you are measuring the pH of your cosmetic products such as creams, lotions and shampoos, you need to dilute the product in distilled water (1:10 dilution), blend the mixture well and then measure the pH. We recommed that you make it your habit to always make a 10% dilution even when you want to measure the pH of a product such as a tonic or micellar water.
Dipping your elecrode or your pH indicator paper directly to the undiluted product does not only provide you with inexact results, it can even damage your electrode.
3- pH is temperature dependant. Wait till your samples reach the room temperature and use room temperature water for your dilutions. Although most modern pH-meters are equipped with a temperature sensor, if there are extreme temperature fluctuations in your lab (icy cold in winter and hot in summer) you may even need to thermostate your samples for a few minutes before measurement.
pH adjustment
Now that you've measurured the pH of the product, one of these 3 possibilities are in front of you:
- The pH of the product is exactly in the range that you want and in the range that your preservative system has the highest efficiency.
Bingo. This is like winning in Lottery. Lucky you if this is the case but it happens very seldom specially when you're working with a new preservative system or a new emulsifier
- The pH of the product is higher than your desired range and you need to reduce it.
Citric acid and lactic acid are the most suitable acids to reduce the pH of a cosmetic product.
Both of them could be prepared quite synthetically or by fermentation. Originally, citric acid is the acid found in lemon juic and lactic acid is the one present in sour milk (it is the product of fermentation of sugar. Your kefir grains ferment the sugar present in milk (or in water/sugar blend) and produce lactic acid and CO2.
Citric acid is usually sold as a powder (anhydrous or hydrated) or as a solution. It is much cheaper than lactic acid. In addition to reducing the pH, citric acid is a chelator (binding metal ions) and can enhance the performance of the preservative .
Lactid acid, although originally a powder, is commercially available as a 80% solution (this is the max. available cncentration commercially available). It is much more expensive that citric acid and in 80% concentration it could be quite corrosive (handle with care, avoid contact with skin).
Lactic acid, in addition to reducing the pH has exceptional moisturizing properties. It is an AHA (alpha-hydroxy acid) and the very same acid used in many chemical peelings.
Which one you use (citric acid or lactic acid) depends on your budget, the availability of the ingredient and your preferences. Both of them reduce the pH. We generally use lactic acid in our laboratory for its additional moisturizing benefits.
Do not use vinegar, lemon juice or household cleaners to reduce the pH
Don't laugh, but this is the advice you can find in some DIY recipes.
How to:
This is the part that unnerves most of the beginners. If you are working with a new formulation, you probably need a few trials to adjust the pH to the range you need. After the second or third trial, you will have an approximate idea of how much acid you need to land on the point you want. Do make notes of every single step you make. This will spare you some repetitions in your future trials.
We usually have stock solutions of different dilutions in our lab. We have 80% lactic acid that we dilute (in distilled water) to 40%, 20% and 10% dilutions. You can keep this dilutions about one week (in a closed container) without any risk of contamination (the pH is so low that there is no risk of contamination but we usually re make our dilutions after one week)
We keep our stock solutions in dropper bottles like this. It makes the dosage very easy and reduces the risk of contamination of your acid. (this is just a suggestion and the way we do it, not a must)
Depending on the pH and how much higher it is than your desired pH, you may need a few drops of an acid. We keep different stock solutions so that we use just a few drops for each 100 gr trial batch. It may take you some time but after a few trials you'll know whether you'll need a 10% solution or a 80%. (It sounds more complicated than it really is).
What if you pass the point?
Specially at the beginning, it is possible that you add too much acid and reduce the pH lower than what you wished. What to do know?
In worst case, you may reduce the pH to a too low point and some of your ingredients that are pH sensitive might degrade or fall out of the solution and precipitate. This is why we always recommend working with small batches at the beginning and gradually scaling up. Discarding a 50 ml batch is not as painful as discarding a 1 kg batch.
If however, you do not reduce the pH too low and you know that none of your ingredients would sediment but still you need to increase the pH, let's say you've reduced the pH to 5,0 but your aim is 5,5, you'll need a base to increase the pH.
- The pH of the product is lower than your desired range and you need to increase it.
Obviously, you'll need a base to increase the pH of the product.
- Baking soda (sodium bicarbonate, known as sodium bicarb) is the most popular base among DIY crafters. It is easily available and easy to use. We usually do not recommend this ingredient but if this is the only available base to you, go ahead and apply it.
Use a food, pharmaceutical or cosmetic grade ingredient and not a household grade for cosmetic applications. Household grade has impurities that you don't want to have in your products
- NaOH or lye is another base to increase the pH of your products. NaOH is called caustic soda and the concentrated form is indeed very caustic and aggressive to the skin, to your clothings and to many surfaces. If you are a soap maker, you're certainly aware of the hassle of working with sodium hydroxide.
Read our previous post about the Safe handling of NaOH
Since you are going to use just tiny amounts of this ingredients to reduce the pH, there would be no residues of it in your finished product and you don't need to be concerned about the safety or harshness of your product.
However, it is a little bit difficult (you need to be more careful) to work with a concentrated lye solution and to make a dilution at the first point, specially if you have never made a soap. It is not always easy to get access to a high purity sodium hydroxide . Most chemical companies do not sell to individuals and private persons and some online handlers want to first know that you are qualified to work with NaOH.
In any case, if you are lucky enough to find a reliable handler who is ready to sell to you, or you have a generous friend who us ready to share with you, you can use NaOH to increase the pH of your products.
Both baking soda and NaOH (lye) solutions are very susceptible to moisture and oxygen. They absorb carbon dioxide and their pH reduces upon storage. Keep your samples in well tight bottles and prepare your stocks so that you can use them up in max. one week.
Do not use any NaOH from hardware store or any drain cleaner, even if they bottle is as 100% pure, the purity requirements are quite different for industrial products and for home cleaners compared to food, pharmaceuticals or cosmetics.
- L-Arginine is an essential or semi-essential amino acid necessary for protein synthesis. It has been used as dietary supplement and in many pharamceuticals (but this is out of our concept). It is a crystalline white product and when you dilute it in water (10%) it has a pH between 10,5-12. Arginine is prepared by fermentation and is a natural ingredient and suitable for vegan formulations.
This is a suitable base to increase the pH of your cosmetic products and it is quite easy (and safe) to work with. If you have any apathy , dislike or considerations about baking soda and NaOH, this is the mildest way to increase the pH of your products.
How to:
Just like decreasing pH, you'll need different dilutions of a base so that you can use tiny amounts to increase the pH of your product.
We usually have 50%, 25% and 10% stock solutions of NaOH (you can do the same with baking soda) and 10% and 5% dilutions of arginine for increasing the pH in our lab. In some cases (after many trial and horror sessions) we add l-arginine as a powder to the product.
What if you pass the point?
Most ingredients are more susceptible to a high pH than to a low pH. Always try to add your base in small aliquots so that you do not jump to a pH of 9. Some certain ingredients will immediately react to a high pH and will sediment (MgSO4 is one of them). In this case, you'll need to discard your batch and redo your experiment. If you are lucky and none of your ingredients reacts to high pH, you'll still need to reduce the pH to your desired point. Go back to the reducing pH by adding and acid in the previous section.
I hope I could help you with this post and answer some of your questions. If you still have any questions and uncertainties, don't hesitate to send me your questions either per mail or on our Facebook page.