
Donnerstag, 17. August 2017
pH measurement in cosmetic lab: why we dilute samples?

pH measurement and adjustment is the alpha and omega of making stable and safe cosmetic products but it seems that only a few students pay enough attention to it.
We have written several blog posts about the importance and necessity of pH measurement in the past and can not help repeating this issue again and again.
Read our past blog posts about the importance of pH measurement:
How to protect your "natural" preservatives from deactivation
What you need to know about pH measurement
How to use a portable pH-meter
How to adjust the pH of your cosmetic products
pH adjustment is not only important to guarantee the integrity of your product, it is a legal requirement (no matter where you are located) that your products should be safe to consumer. A too low or too high pH not only might deactivate your preservative system and affect the overall stability and efficacy of the product, it might cause harm to the consumer.
Now that we all agree on the importance and necessity of pH adjustment, let's go to "how-to" of pH measurement.
Important:
pH is an expression of acidity and alkalinity and the number of hydronium (H3O+ or in simple words H+) ions.
Where there are no ions, there is no pH. This means that for oils, butters and other lipophilic material, there is no pH to be measured. This is the same for your lipophilic finished products such as balms, butters, oils and lipophilic serums.
For raw material:
Measurement of the pH of your raw material (and your distilled or deionized water) is one important step in your quality control lab. The pH is mentioned on the COA and technical data sheet of almost all of water containing (and water soluble) raw material. Have a look at the TDS of some of your raw material. For most of them a pH of a 5% or 10% dilution is mentioned. In some exceptional cases (such as hydrosols) the pH of the raw material is measured directly and without dilution.
Finished products:
We generally recommend a 10% dilution (10 gr product + 90 gr distilled water) for pH measurement.
Most of new students (and unfortunately some self-named experts and so-called tutors) do not like the idea of preparing a 10% solution (it is too time consuming isn't it? And believe me, after each pH measurement you'll have tens of beakers to cleans and rinse).
Why a dilution?
1- Working with indicator paper
Although indicator papers are not exact for a cosmetic lab, they are the first and only choice for the very beginners. We don't recommend using indicator papers for pH measurement (except the ones we sell ![]() ) but we don't want to force new students to go and purchase a pH-meter (although in a long run the pH-meter is much more economic than these papers).
) but we don't want to force new students to go and purchase a pH-meter (although in a long run the pH-meter is much more economic than these papers).
Anyway, try to dip your paper in a lotion or in a shampoo and then try to read what it indicates? NOTHING
Unless you are working with a very dilute product such as a body splash or a facial tonic, the paper doesn't show you anything when dipped in a concentrated (and high viscosity) product.
2- Working with a pH-Meter and a glass electrode
Even if you are not a chemist, there are some interesting basic articles about the principles of pH measurements and how a glass electrode is built. This helps you understand the anatomy of the pH electrode and learn how you can guarantee the maintenance and a long-life for your electrode:
https://www.ysi.com/ysi-blog/water-blogged-blog/2015/03/anatomy-of-a-ph-electrode-glass-ph-probes-part-1-of-4
http://hannainst.com/resources/ph/guides/ph-measurement-checklist--hanna-instruments%20.pdf
http://hannainst.com/resources/ph/guides/ph-electrode-maintenance-guide--hanna-instruments.pdf
http://www.emerson.com/documents/automation/68432.pdf
The principle of pH measurement with a glass electrode is based on ion exchenge between the inner solution and the sample you are measuring
This means, you need a certain flow of ions for an exact pH measurement (this is why you should stir the solution during measurement). Dipping the electrode in a concentrated and high viscosity sample (emulsions, shampoo, gel etc.) does not allow adequate ion flow between the sample and the electrode solution. In other words: no precise measurement.
Apart from that, the more concentrated sample you use, the higher the chances of killing your electrode by reducing the porosity of the glass membrane.
Another reason (this is a secondary reason) for using dilutions is economy. In a manufacturing facility and when making a 5000 tons batch you can easily pick 50 ml of the product for pH measurement but when making small samples in your development lab, you usually work with 50-200 gr batch size. Removing some 30-50 ml for a pH measurement is almost impossible (well, there would be nothing left for other tests and for application of the sample) and dipping the electrode in the whole batch is a No-GO.
Disclaimer: There are some exceptional and high-end electrodes which you can directly immerse in the original sample (even high viscosity samples) but I think you would rather wash a few beakers and purchase an affordable pH electrode than invest 10 times the price in the electrode to skip diluting samples and washing beakers ![]() (Your lab, your choice).
(Your lab, your choice).
One consideration from new students is:
I want to measure the pH of my product (that comes into contact with the skin). When the pH of a 10% cream is for example 5.2, what would be the pH of the original cream on the skin?
Well, to answer your question and to calm your minds we ran an experiment and measured the pH of a 50% dilution (not the original emulsion because we didn't want to kill our electrode) emulsion and then diluted this emulsion subsequently (In each step we diluted the emulsion to 50%).
This is the result:
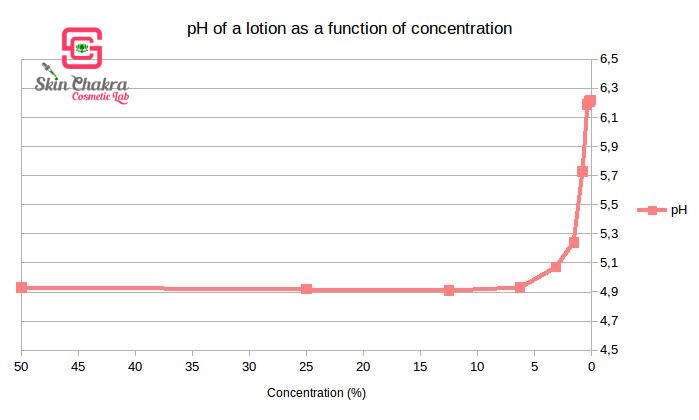
Looking at the above diagram, you see that the pH of the 50% emulsion is 4,9. (The pH of our distilled water was 6,2)
We have diluted the emulsion to 25%, 12,5%, 6,25% and so on and you can see that the pH of the samples remain the same up to a dilution of 2,5%. From here, the pH increases until it reaches the pH of distilled water (which is our solvent or diluent).
I hope this diagram can comfort your mind that a 10% dilution of a cream (or a shampoo or shower gel) has the same pH as the undiluted and original product.
I hope this blog post can asnwer your questions about the necessity of making a 10% dilution when measuring the pH of your products.
Don't hesitate to send us your comments and questions either per mail or to our Facebook page

